Antídotos
Hernando Andrés Olaya Acosta
Médico y Cirujano. Especialista en Toxicología y Farmacología Clínica
Toxicólogo Clínico, Clínica del Country – Hospital Infantil Universitario de San José. Bogotá D.C
Profesor Asistente, Pontificia Universidad Javeriana. Bogotá D.C
Miembro de la Asociación de Toxicología Clínica Colombiana –ATCC
Miembro de la Asociación Colombiana de Medicina Interna –ACMI
Miguel Antonio Tolosa Rodríguez
Médico y Cirujano Especialista en Toxicología Clínica
Toxicólogo Clínico, Hospital Infantil Universitario de San José. Bogotá D.C
Profesor Asistente, Fundación Universitaria Ciencia de la Salud – FUCS
Miembro de la Asociación de Toxicología Clínica Colombiana –ATCC
Miembro de la Academia Americana de Toxicología Clínica –AACT
Verónica Manosalva Jiménez
Médica y Cirujana
Residente de Toxicología Clínica de la Fundación Universitaria Ciencia de la Salud –FUCS
Miembro de la Asociación de Toxicología Clínica Colombiana -ATCC
Introducción
Según reporte epidemiológico del Sistema de Vigilancia de Eventos en Salud Pública – Sivigila, en el año 2015 se reportaron al sistema 14.477 casos de intoxicación con intencionalidad suicida, siendo el 53.5% intoxicaciones con medicamentos y 34.4% con plaguicidas población1.
La población más afectada fueron jóvenes entre 15 y 24 años1. En ese mismo año, fueron notificados a Sivigila 33.503 casos de intoxicaciones por sustancias químicas (principalmente con medicamentos), lo que representó un aumento del 5.4% de casos respecto al año anterior (1).
Por otra parte, la Asociación Americana de Centros de Control de Envenenamiento (APCC por sus siglas en inglés) anualmente reporta en promedio 2.400.000 exposiciones a tóxicos en humanos y 1300 muertes relacionadas con intoxicaciones2. Sin embargo, solo algunos pacientes requieren hospitalización y no a todos se les administra antídoto, debido a que estos se utilizan bajo criterios especiales y no siempre se cuenta con ellos. (Lea también: Enfermedades Transmitidas por Alimentos)
La poca disponibilidad de antídotos:
Es un fenómeno mundial que se ha atribuido a costos, dificultades en importación o en fabricación, medicamento disponible en una forma farmacéutica inadecuada para tratamiento de la intoxicación, poco uso de algunos o dificultades regulatorias3, 4. Por ejemplo en Inglaterra solo alrededor del 50% de los hospitales tiene disponible los antídotos recomendados para un servicio de urgencias5.
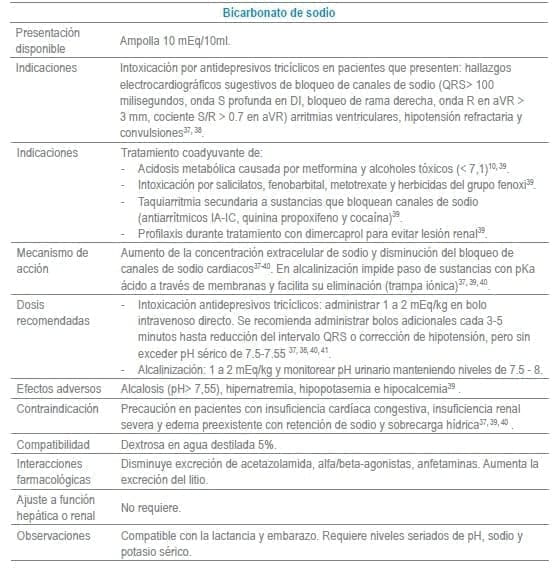
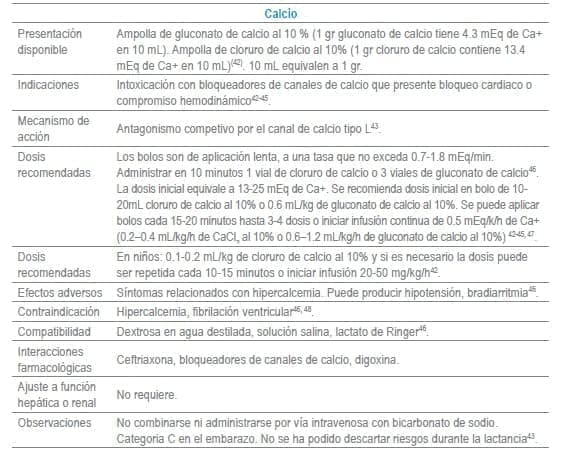
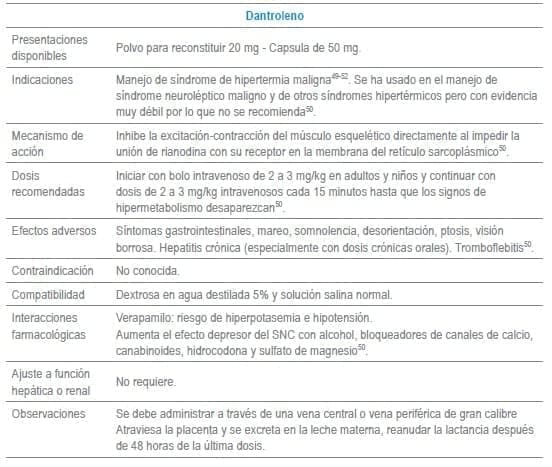
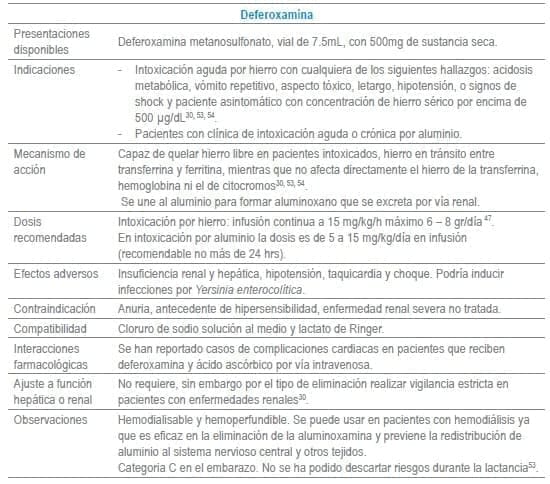
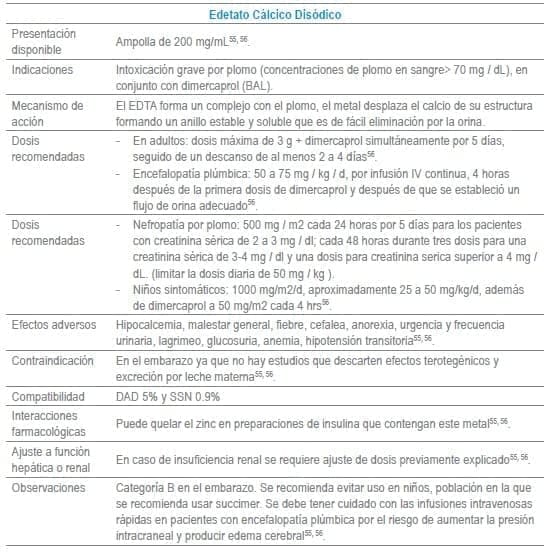
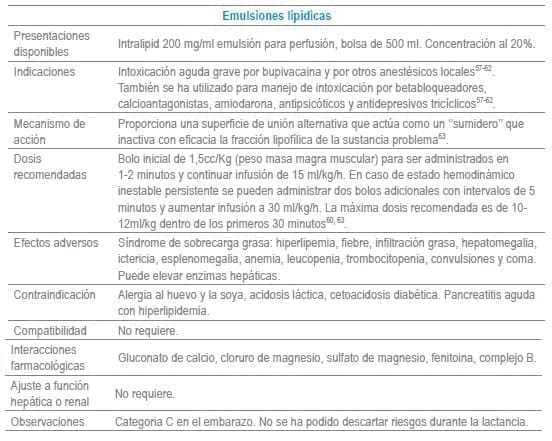
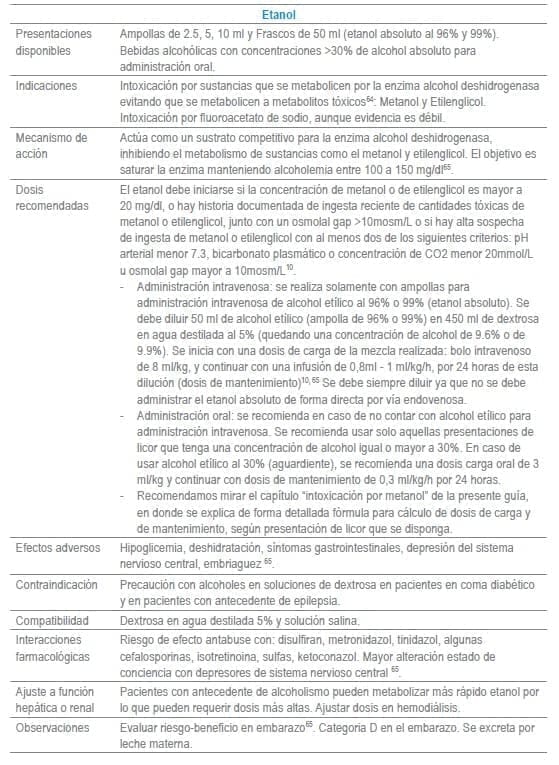
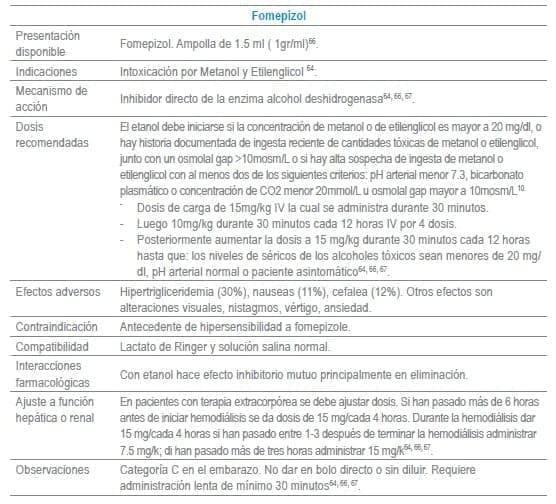
En este capítulo se incluye tabla con los antídotos más frecuentemente usados en servicios de urgencias a nivel mundial, infortunadamente algunos de los mencionados no se encuentran disponibles o tienen acceso muy limitado en Colombia al igual que en otros países de América Latina. La tabla como guía práctica, incluye indicación, dosis, forma de administración, seguimiento clínico, precauciones y eventos adversos frecuentes de cada uno de ellos.
Adicionalmente se debe tener en cuenta que en algunas situaciones es el escenario clínico quien determina la decisión no solo del uso de un antídoto, sino que también su vía de administración y el cálculo de la dosis. La indicación puede ser variable dependiendo del tóxico y del momento clínico de la administración.
Se recomienda que, ante cualquier sospecha de intoxicación o envenenamiento, siempre se cuente con el apoyo de líneas de toxicología y de centros hospitalarios que cuenten con toxicólogos clínicos.
Definiciones a tener en cuenta:
- Agonista: molécula o fármaco que se une al receptor y activa señalización intracelular6.
- Antagonista: molécula o fármaco que se une al receptor, pero no desencadena la generación de una señal6.
- Antídoto: agente que aumenta la dosis letal media de una toxina o simplemente un agente que invierte o neutraliza un veneno7.
- Quelante: del latín chela o garra, molécula con la propiedad de unirse con el metal y formar complejos atóxicos que facilitan la excreción del organismo8.
Antídotos seleccionados para manejo de urgencias
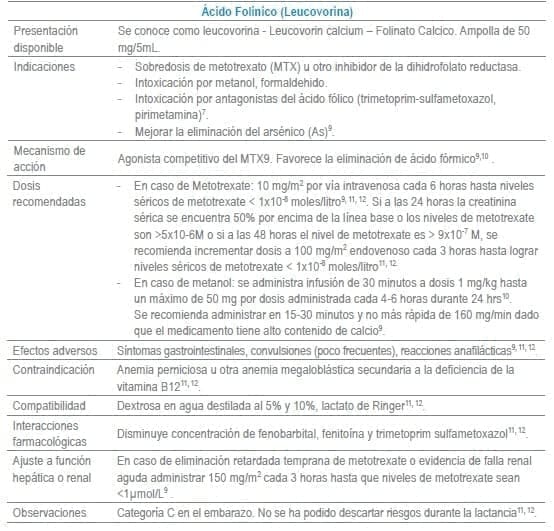

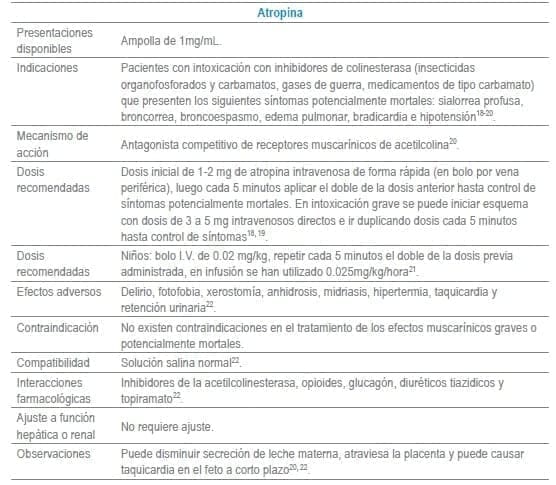
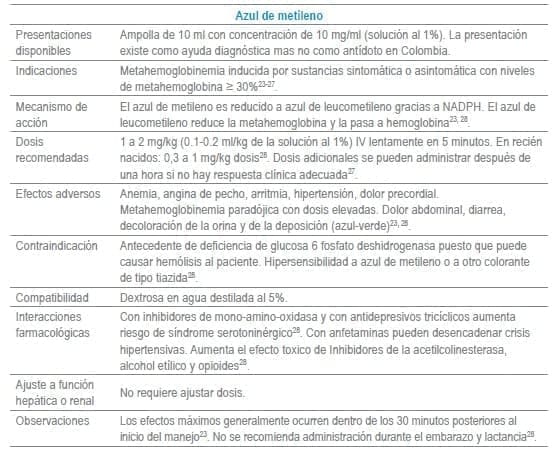
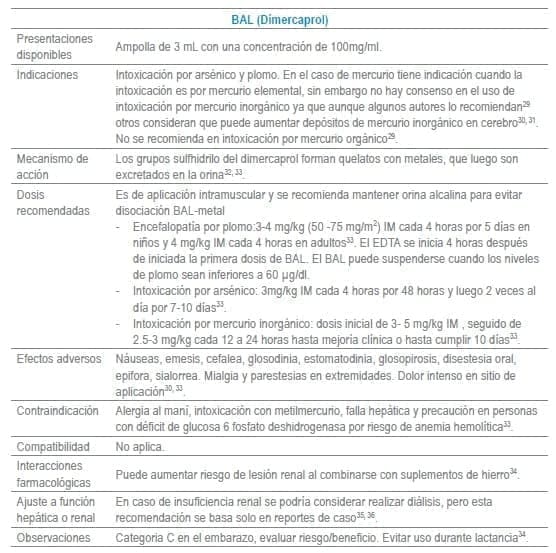
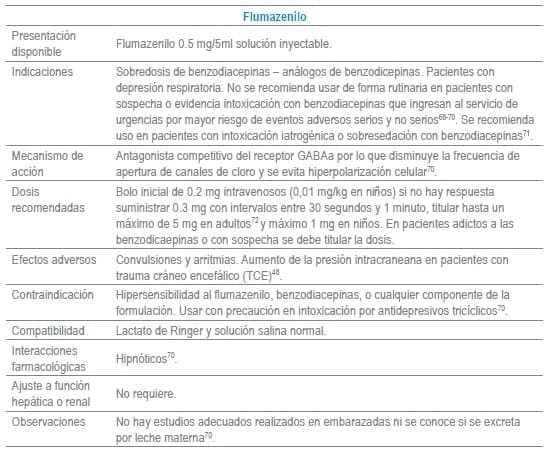
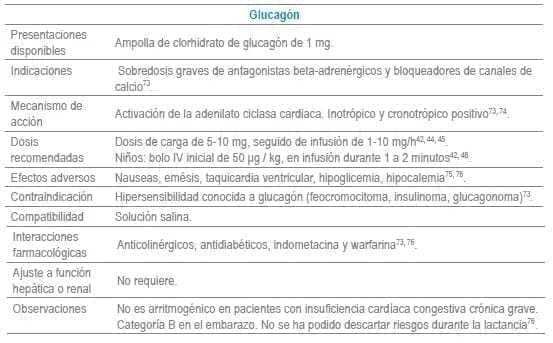
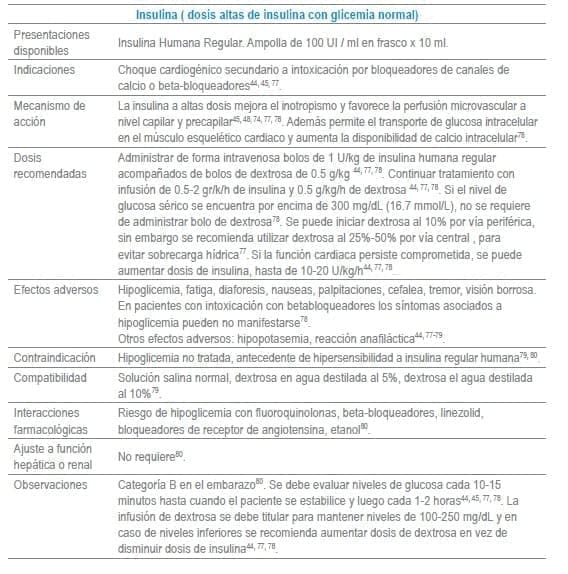
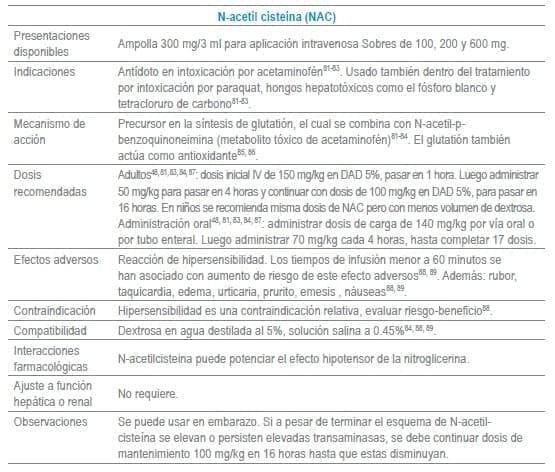
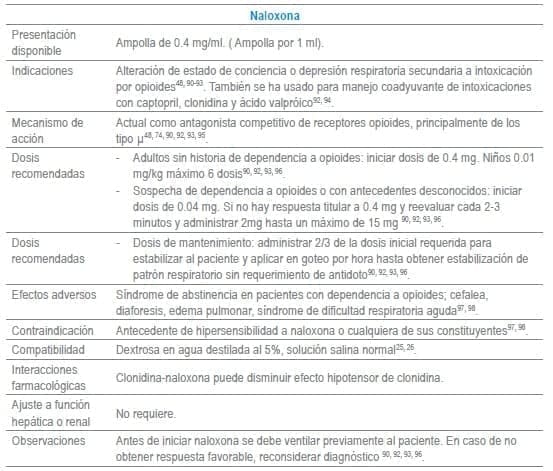
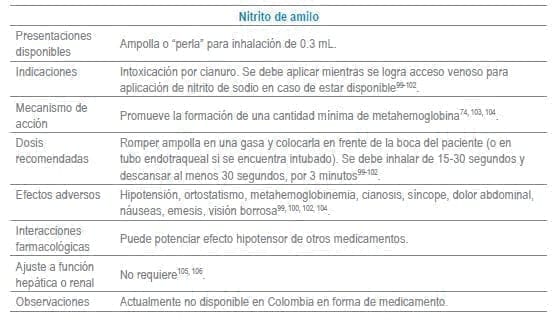
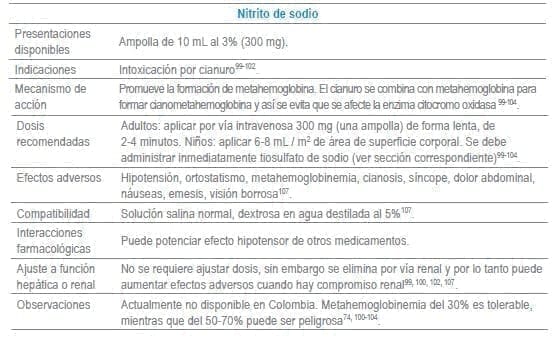
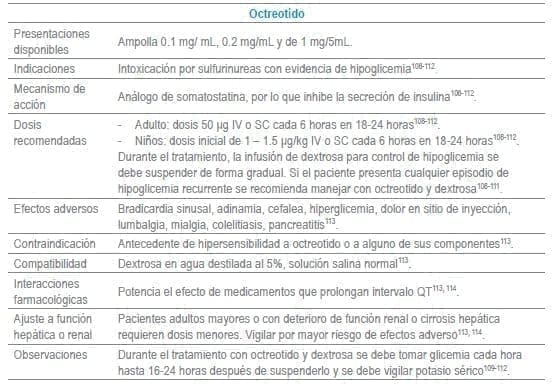
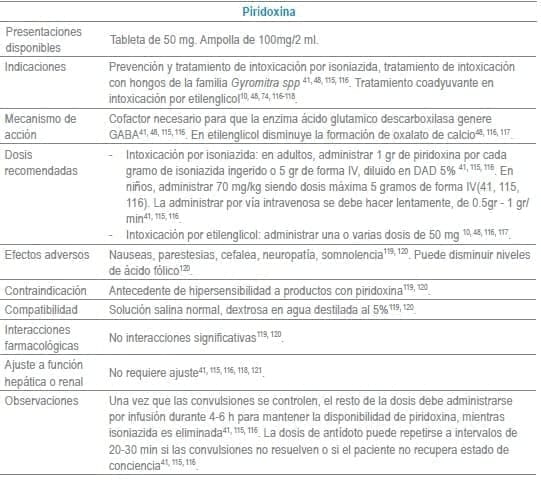
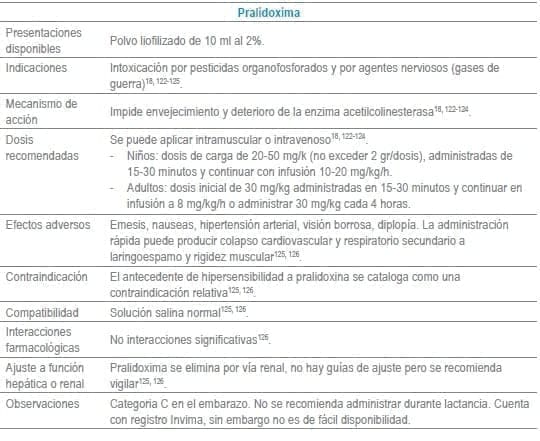
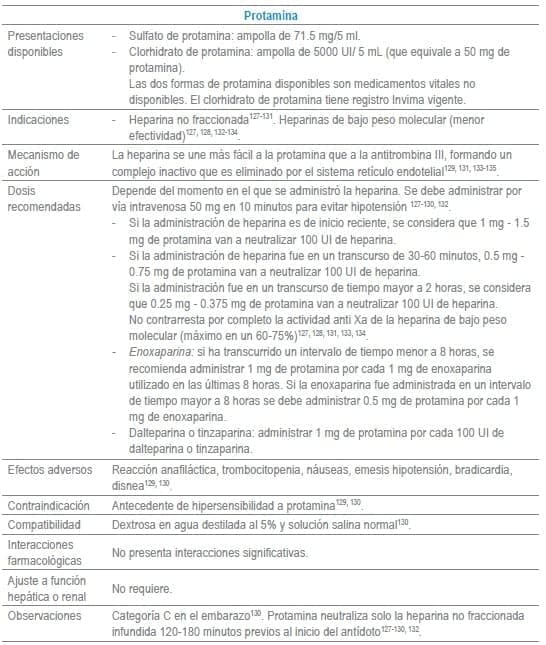
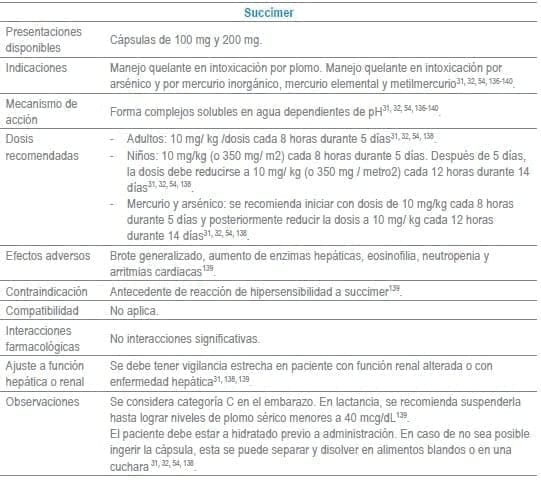

Referencias
- 1. Dirección de Vigilancia y Análisis del Riesgo en Salud Pública, Instituto Nacional de Salud. República de Colombia. Semana epidemiológica número 52 de 2015 (27 dic. al 02 ene.). Colombia2015.
- 2. Marraffa JM, Cohen V, Howland MA. Antidotes for toxicological emergencies: a practical review. Am J Health Syst Pharm. 2012;69(3):199-212.
- 3. Galvao TF, Bucaretchi F, De Capitani EM, Pereira MG, Silva MT. [Antidotes and medicines used to treat poisoning in Brazil: needs, availability and opportunities]. Cad Saude Publica. 2013;29 Suppl 1:S167-77.
- 4. Mazer-Amirshahi M, Hawley KL, Zocchi M, Fox E, Pines JM, Nelson LS. Drug shortages: Implications for medical toxicology. Clin Toxicol (Phila). 2015;53(6):519-24.
- 5. Mayor S. Only half of British acute care hospitals stock all essential antidotes to poisons, survey shows. Bmj. 2012;345:e5491.
- 6. Blumenthal DK, Garrison JC. Pharmacodynamics: Molecular Mechanisms of Drug Action. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 12e. New York, NY: McGraw-Hill Education; 2011.
- 7. Paloucek FP. Effective Use of Antidotes. In: Leikin JB, Paloucek FP, editors. Poisoning and Toxicology Compendium. Firts ed. Canada: Lexi-Comp, Incorporated; 1998. p. 1014-6.
- 8. Byrns MC, Penning TM. Environmental Toxicology: Carcinogens and Heavy Metals. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 12e. New York, NY: McGraw-Hill Education; 2011.
- 9. Howland MA. Folates: Leucovorin (Folinic Acid) and Folic Acid. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 693-7.
- 10. Kruse JA. Methanol and ethylene glycol intoxication. Crit Care Clin. 2012;28(4):661-711.
- 11. Leucovorin Calcium. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 12. Leucovorin: Drug information. En: UptoDate® [Internet]. Wolters Kluwer Health. 2016 [cited Abril 2016]. Disponible en: http://www.uptodate.com.ez.urosario.edu.co/.
- 13. Roberts DM, Gallapatthy G, Dunuwille A, Chan BS. Pharmacological treatment of cardiac glycoside poisoning. Br J Clin Pharmacol. 2016;81(3):488-95.
- 14. Chan BS, Buckley NA. Digoxin-specific antibody fragments in the treatment of digoxin toxicity. Clin Toxicol (Phila). 2014;52(8):824-36.
- 15. Kanji S, MacLean RD. Cardiac glycoside toxicity: more than 200 years and counting. Crit Care Clin. 2012;28(4):527-35.
- 16. Howland MA. Antidotes in Depth. Digoxin-Specific Antibody Fragments. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 904-8.
- 17. Drug Monographs. Digoxin Immune Fab. En: Access Medicine [Internet]. McGraw-Hill Medical/Education. 2016 [cited Abril 2016]. Disponible en: http://accessmedicine.mhmedical.com.ez.urosario.edu.co/drugs.aspx?gbosID=131134.
- 18. King AM, Aaron CK. Organophosphate and carbamate poisoning. Emerg Med Clin North Am. 2015;33(1):133-51.
- 19. Eddleston M, Chowdhury FR. Pharmacological treatment of organophosphorus insecticide poisoning: the old and the (possible) new. Br J Clin Pharmacol. 2016;81(3):462-70.
- 20. Howland MA. Antidotes in Depth. Atropine. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1425-8.
- 21. Castaño PA. Intoxicación por inhibidores de colinesterasas. En: Peña LM, Zuluaga AF editores. Protocolos de manejo del paciente intoxicado. Primera edición. Dirección Seccional de Salud y Protección Social de Antioquía. Medellín, Antioquia. Disponible en: https://www.dssa.gov.co/index.php/descargas/1016-protocolos-manejode-intoxicadosversionabreviada2011/file.
- 22. Atropine Sulfate. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 23. Howland MA. Antidotes in Depth. Methylene Blue. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/ Medical; 2014. p. 1631-3.
- 24. Lo JC, Darracq MA, Clark RF. A review of methylene blue treatment for cardiovascular collapse. J Emerg Med. 2014;46(5):670-9.
- 25. Ashurst J, Wasson M. Methemoglobinemia: a systematic review of the pathophysiology, detection, and treatment. Del Med J. 2011;83(7):203-8.
- 26. Schirmer RH, Adler H, Pickhardt M, Mandelkow E. “Lest we forget you–methylene blue…”. Neurobiol Aging. 2011;32(12):2325.e7-16.
- 27. Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J. 2011;104(11):757-61.
- 28. Methylene Blue. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com. 29. Sue Y-J. Mercury. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1250-8.
- 30. Aaseth J, Skaug MA, Cao Y, Andersen O. Chelation in metal intoxication–Principles and paradigms. J Trace Elem Med Biol. 2015; 31:260-6.
- 31. Cao Y, Skaug MA, Andersen O, Aaseth J. Chelation therapy in intoxications with mercury, lead and copper. J Trace Elem Med Biol. 2015; 31:188-92.
- 32. Kosnett MJ. The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol. 2013;9(4):347-54.
- 33. Howland MA. Antidotes in Depth. Dimercaprol (British Anti-Lewisite or BAL). In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1181-3.
- 34. Dimercaprol. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 35. Mathieu D, Mathieu-Nolf M, Germain-Alonso M, Neviere R, Furon D, Wattel F. Massive arsenic poisoning–effect of hemodialysis and dimercaprol on arsenic kinetics. Intensive Care Med. 1992;18(1):47-50.
- 36. Ellis EN, Brouhard BH, Lynch RE, Dawson EB, Tisdell R, Nichols MM, et al. Effects of hemodialysis and dimercaprol in acute dichromate poisoning. J Toxicol Clin Toxicol. 1982;19(3):249-58.
- 37. Bruccoleri RE, Burns MM. A Literature Review of the Use of Sodium Bicarbonate for the Treatment of QRS Widening. J Med Toxicol. 2016;12(1):121-9.
- 38. Yates C, Galvao T, Sowinski KM, Mardini K, Botnaru T, Gosselin S, et al. Extracorporeal treatment for tricyclic antidepressant poisoning: recommendations from the EXTRIP Workgroup. Semin Dial. 2014;27(4):381-9.
- 39. Wax PM. Antidotes in Depth. Sodium Bicarbonate. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/ Medical; 2014. p. 528-35.
- 40. Liebelt EL. Cyclic Antidepressants. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 972-82.
- 41. Sharma AN, Hoffman RJ. Toxin-related seizures. Emerg Med Clin North Am. 2011;29(1):125-39.
- 42. Arroyo AM, Kao LW. Calcium channel blocker toxicity. Pediatr Emerg Care. 2009;25(8):532-8; quiz 9-40.
- 43. Howland MA. Antidotes in Depth. Calcium. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1330-3.
- 44. Graudins A, Lee HM, Druda D. Calcium channel antagonist and betablocker overdose: antidotes and adjunct therapies. Br J Clin Pharmacol. 2016;81(3):453-61.
- 45. St-Onge M, Dube PA, Gosselin S, Guimont C, Godwin J, Archambault PM, et al. Treatment for calcium channel blocker poisoning: a systematic review. Clin Toxicol (Phila). 2014;52(9):926-44.
- 46. Calcium Gluconate. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 47. Olaya A. Tablas de Toxicología. In: Severiche D, editor. Medicina práctica: tablas diagnósticas y terapéuticas en urgencias UCI y medicina interna. 1ra ed. Colombia: Distribuna Editorial; 2012. p. 262.
- 48. Smith SW. Drugs and pharmaceuticals: management of intoxication and antidotes. Exs. 2010; 100:397-460.
- 49. Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K. Malignant hyperthermia: a review. Orphanet J Rare Dis. 2015; 10:93.
- 50. Sutin KM. Antidotes in Depth. Dantrolene Sodium. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 955-7.
- 51. Musselman ME, Saely S. Diagnosis and treatment of drug-induced hyperthermia. Am J Health Syst Pharm. 2013;70(1):34-42.
- 52. Correia AC, Silva PC, da Silva BA. Malignant hyperthermia: clinical and molecular aspects. Rev Bras Anestesiol. 2012;62(6):820-37.
- 53. Howland MA. Antidotes in Depth. Deferoxamine. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 623-7.
- 54. Smith SW. The role of chelation in the treatment of other metal poisonings. J Med Toxicol. 2013;9(4):355-69.
- 55. Calcium Disodium Versenate. NDA 8-922/S-016. Graceway Pharmaceuticals, LLC Bristol, TN 37620 By: Luitpold Pharmaceuticals, Inc. Shirley, NY. Disponible en: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/008922s016lbl.pdf.
- 56. Howland MA. Antidotes in Depht. Edetate Calcium Disodium (CaNa2EDTA) In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1241-44.
- 57. ACMT Position Statement: Guidance for the Use of Intravenous Lipid Emulsion. J Med Toxicol. 2016.
- 58. Hayes BD, Gosselin S, Calello DP, Nacca N, Rollins CJ, Abourbih D, et al. Systematic review of clinical adverse events reported after acute intravenous lipid emulsion administration. Clin Toxicol (Phila). 2016;54(5):365-404.
- 59. Hoegberg LC, Bania TC, Lavergne V, Bailey B, Turgeon AF, Thomas SH, et al. Systematic review of the effect of intravenous lipid emulsion therapy for local anesthetic toxicity. Clin Toxicol (Phila). 2016;54(3):167-93.
- 60. Lam SH, Majlesi N, Vilke GM. Use of Intravenous Fat Emulsion in the Emergency Department for the Critically Ill Poisoned Patient. J Emerg Med. 2016.
- 61. Levine M, Hoffman RS, Lavergne V, Stork CM, Graudins A, Chuang R, et al. Systematic review of the effect of intravenous lipid emulsion therapy for non-local anesthetics toxicity. Clin Toxicol (Phila). 2016;54(3):194-221.
- 62. Bania TC. Antidotes in Depth. Intravenous Fat Emulsion. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 931-7.
- 63. Ozcan MS, Weinberg G. Intravenous lipid emulsion for the treatment of drug toxicity. J Intensive Care Med. 2014;29(2):59-70.
- 64. McMartin K, Jacobsen D, Hovda KE. Antidotes for poisoning by alcohols that form toxic metabolites. Br J Clin Pharmacol. 2016;81(3):505-15.
- 65. Howland MA. Antidotes in Depth. Ethanol. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1369-72.
- 66. Howland MA. Antidotes in Depht. Fomepizole. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1364-68.
- 67. Brent J, McMartin K, Phillips S, Aaron C, Kulig K. Fomepizole for the treatment of methanol poisoning. N Engl J Med. 2001;344(6):424-9.
- 68. Penninga EI, Graudal N, Ladekarl MB, Jurgens G. Adverse Events Associated with Flumazenil Treatment for the Management of Suspected Benzodiazepine Intoxication–A Systematic Review with Meta-Analyses of Randomised Trials. Basic Clin Pharmacol Toxicol. 2016;118(1):37-44.
- 69. Sivilotti ML. Flumazenil, naloxone and the ‘coma cocktail’. Br J Clin Pharmacol. 2016;81(3):428-36.
- 70. Howland MA. Antidotes in Depth. Flumazenil. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1013-7.
- 71. Yonel Z, Asuni A, Taneja P. Defining Over-Sedation: Literature Review and National Survey of Dental Hospitals Within the United Kingdom. SAAD Dig. 2016; 32:28-33.
- 72. Olaya A. Tablas de Toxicología. In: Severiche D, editor. Medicina práctica, tablas diagnósticas y terapéuticas en urgencias, UCI y medicina interna. Colombia: Distribuna Editorial; 2012. p. 262.
- 73. Howland MA. Antidotes in Depth. Glucagon. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 870-3.
- 74. Betten DP, Vohra RB, Cook MD, Matteucci MJ, Clark RF. Antidote use in the critically ill poisoned patient. J Intensive Care Med. 2006;21(5):255-77.
- 75. Mann S. Management of calcium channel blocker overdose. Emerg Nurse. 2014;21(10):36-9.
- 76. Glucagon Hydrochloride. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 77. Bartlett D. beta-Blocker and Calcium Channel Blocker Poisoning: High-Dose Insulin/Glucose Therapy. Crit Care Nurse. 2016;36(2):45-50.
- 78. Stellpflug SJ, Kerns W. Antidotes in Depth. High-Dose Insulin Euglycemia. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed: McGraw-Hill Education /Medical; 2014. p. 851-5.
- 79. Drug Monographs. Insulin Regular. En: Access Medicine [Internet]. Mc-Graw Hill Medical/Education. 2016 [cited Abril 2016]. Disponible en: http://accessmedicine.mhmedical.com/drugs.
- 80. Insulin Human Regular. In: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 81. Gosselin S, Juurlink DN, Kielstein JT, Ghannoum M, Lavergne V, Nolin TD, et al. Extracorporeal treatment for acetaminophen poisoning: recommendations from the EXTRIP workgroup. Clin Toxicol (Phila). 2014;52(8):856-67.
- 82. Bass S, Zook N. Intravenous acetylcysteine for indications other than acetaminophen overdose. Am J Health Syst Pharm. 2013;70(17):1496-501.
- 83. Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28(4):499-516.
- 84. Hendrickson RG, A HM. Antidotes in Depth. N-Acetylcysteine. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 465-72.
- 85. Gil HW, Hong JR, Jang SH, Hong SY. Diagnostic and therapeutic approach for acute paraquat intoxication. J Korean Med Sci. 2014;29(11):1441-9.
- 86. Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72(5):745-57.
- 87. Bateman DN, Dear JW, Thanacoody HK, Thomas SH, Eddleston M, Sandilands EA, et al. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomized controlled trial. Lancet. 2014;383(9918):697-704.
- 88. Acetylcysteine: Drug information En: UptoDate® [Internet]. Wolters Kluwer Health. 2016 [cited Abril 2016]. Disponible en: http://www.uptodate.com.ez.urosario.edu.co/.
- 89. Acetylcysteine. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 90. Kim HK, Nelson LS. Reducing the harm of opioid overdose with the safe use of naloxone: a pharmacologic review. Expert Opin Drug Saf. 2015;14(7):1137-46.
- 91. Davis CS, Southwell JK, Niehaus VR, Walley AY, Dailey MW. Emergency medical services naloxone access: a national systematic legal review. Acad Emerg Med. 2014;21(10):1173-7.
- 92. Nelson LS, Howland MA. Antidotes in Depth. Opioid Antagonist. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 511-5.
- 93. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-55.
- 94. Ahmad SA, Scolnik D, Snehal V, Glatstein M. Use of naloxone for clonidine intoxication in the pediatric age group: case report and review of the literature. Am J Ther. 2015;22(1): e14-6.
- 95. Volkow ND, McLellan AT. Opioid Abuse in Chronic Pain—Misconceptions and Mitigation Strategies. N Engl J Med. 2016;374(13):1253-63.
- 96. Larney S, Gowing L, Mattick RP, Farrell M, Hall W, Degenhardt L. A systematic review and meta-analysis of naltrexone implants for the treatment of opioid dependence. Drug Alcohol Rev. 2014;33(2):115-28.
- 97. Drug Monographs. Naloxone. En: Access Medicine [Internet]. Access Medicine. 2016 [cited abril 2016]. Disponible en: http://accessmedicine.mhmedical.com.ez.urosario.edu.co/drugs.
- 98. Naloxone Hydrochloride. In: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 99. Howland MA. Antidotes in Depth. Sodium and Amyl Nitrite. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1612-4.
- 100. Mintegi S, Clerigue N, Tipo V, Ponticiello E, Lonati D, Burillo-Putze G, et al. Pediatric cyanide poisoning by fire smoke inhalation: A European expert consensus. Toxicology Surveillance System of the Intoxications Working Group of the Spanish Society of Paediatric Emergencies. Pediatr Emerg Care. 2013;29(11):1234-40.
- 101. Borron SW, Baud FJ. Antidotes for acute cyanide poisoning. Curr Pharm Biotechnol. 2012;13(10):1940-8.
- 102. Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC. Review article: management of cyanide poisoning. Emerg Med Australas. 2012;24(3):225-38.
- 103. Anseeuw K, Delvau N, Burillo-Putze G, De Iaco F, Geldner G, Holmstrom P, et al. Cyanide poisoning by fire smoke inhalation: a European expert consensus. Eur J Emerg Med. 2013;20(1):2-9.
- 104. Morocco AP. Cyanides. Crit Care Clin. 2005;21(4):691-705, vi.
- 105. Drug Monographs. Amyl Nitrite. En: Access Medicine [Internet]. McGraw-Hill Medical/Education. 2016 [cited Abril 2016]. Disponible en: http://accessmedicine.mhmedical.com.ez.urosario.edu.co/drugs.
- 106. Amyl Nitrite. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 107. Sodium nitrite: Drug information. En: UptoDate® [Internet]. Wolters Kluwer Health. 2016 [cited Abril 2016]. Disponible en: http://www.uptodate.com.ez.urosario.edu.co/.
- 108. Howland MA, Smith SW. Antidotes in Depth. Octreotide. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 738-42.
- 109. Dougherty PP, Lee SC, Lung D, Klein-Schwartz W. Evaluation of the use and safety of octreotide as antidotal therapy for sulfonylurea overdose in children. Pediatr Emerg Care. 2013;29(3):292-5.
- 110. Llamado R, Czaja A, Stence N, Davidson J. Continuous octreotide infusion for sulfonylurea-induced hypoglycemia in a toddler. J Emerg Med. 2013;45(6): e209-13.
- 111. Glatstein M, Scolnik D, Bentur Y. Octreotide for the treatment of sulfonylurea poisoning. Clin Toxicol (Phila). 2012;50(9):795-804.
- 112. Dougherty PP, Klein-Schwartz W. Octreotide’s role in the management of sulfonylurea-induced hypoglycemia. J Med Toxicol. 2010;6(2):199-206.
- 113. Drug Monographs. Octreotide. En: Access Medicine [Internet]. McGraw-Hill Medical/Education. 2016 [cited Abril 2016]. Disponible en: http://accessmedicine.mhmedical.com.ez.urosario.edu.co/drugs.
- 114. Octreotide Acetate. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 115. Chen HY, Albertson TE, Olson KR. Treatment of drug-induced seizures. Br J Clin Pharmacol. 2016;81(3):412-9.
- 116. Howland MA. Antidotes in Depth. Pyridoxine. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 797-9.
- 117. Caravati EM, Erdman AR, Christianson G, Manoguerra AS, Booze LL, Woolf AD, et al. Ethylene glycol exposure: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2005;43(5):327-45.
- 118. Lheureux P, Penaloza A, Gris M. Pyridoxine in clinical toxicology: a review. Eur J Emerg Med. 2005;12(2):78-85.
- 119. Pyridoxine. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 120. Vitamin B6 (pyridoxine): Drug information. En: UptoDate® [Internet]. Wolters Kluwer Health. 2016 [cited abril 2016]. ADisponible en: http://www.uptodate.com.ez.urosario.edu.co/.
- 121. Umapathi T, Chaudhry V. Toxic neuropathy. Curr Opin Neurol. 2005;18(5):574-80.
- 122. Buckley NA, Eddleston M, Li Y, Bevan M, Robertson J. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst Rev. 2011(2): Cd005085.
- 123. Worek F, Thiermann H. The value of novel oximes for treatment of poisoning by organophosphorus compounds. Pharmacol Ther. 2013;139(2):249-59.
- 124. Barelli A, Soave PM, Del Vicario M, Barelli R. New experimental Oximes in the management of organophosphorus pesticides poisoning. Minerva Anestesiol. 2011;77(12):1197-203.
- 125. A HM. Antidotes in Depth. Pralidoxime. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1429-34.
- 126. Pralidoxime Chloride. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 127. Greinacher A, Thiele T, Selleng K. Reversal of anticoagulants: an overview of current developments. Thromb Haemost. 2015;113(5):931-42.
- 128. Gomez-Outes A, Suárez-Gea ML, Lecumberri R, Terleira-Fernandez AI, Vargas-Castrillon E. Specific antidotes in development for reversal of novel anticoagulants: a review. Recent Pat Cardiovasc Drug Discov. 2014;9(1):2-10.
- 129. Howland MA. Antidotes in Depth. Protamine. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. New York, EU: McGraw-Hill Education/Medical; 2014. p. 839-42.
- 130. Protamine sulfate: Drug information. En: UptoDate® [Internet]. Wolters Kluwer Health. 2016 [cited Abril 2016]. Disponible en: http://www.uptodate.com.ez.urosario.edu.co/.
- 131. Suryanarayan D, Schulman S. Potential antidotes for reversal of old and new oral anticoagulants. Thromb Res. 2014;133 Suppl 2: S158-66.
- 132. Khan LM, Al-Harthi SE, Alkreathy HM, Osman AM, Ali AS. Detection of adverse drug reactions by medication antidote signals and comparison of their sensitivity with common methods of ADR detection. Saudi Pharm J. 2015;23(5):515-22.
- 133. van Veen JJ, Maclean RM, Hampton KK, Laidlaw S, Kitchen S, Toth P, et al. Protamine reversal of low molecular weight heparin: clinically effective? Blood Coagul Fibrinolysis. 2011;22(7):565-70.
- 134. Monte AA, Bodmer M, Schaeffer TH. Low-molecular-weight heparin overdose: management by observation. Ann Pharmacother. 2010;44(11):1836-9.
- 135. Nemeno JG, Lee S, Yang W, Lee KM, Lee JI. Applications and implications of heparin and protamine in tissue engineering and regenerative medicine. Biomed Res Int. 2014; 2014:936196.
- 136. Cao Y, Chen A, Jones RL, Radcliffe J, Dietrich KN, Caldwell KL, et al. Efficacy of succimer chelation of mercury at background exposures in toddlers: a randomized trial. J Pediatr. 2011;158(3):480-5. e1.
- 137. Bradberry S, Vale A. Dimercaptosuccinic acid (succimer; DMSA) in inorganic lead poisoning. Clin Toxicol (Phila). 2009;47(7):617-31.
- 138. Howland MA. Antidotes in Depth. Succimer (2,3-Dimercaptosuccinic acid). In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1235-8.
- 139. Succimer. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
- 140. Jin Y, Yu F, Liao Y, Liu S, Liu M, Xu J, et al. Therapeutic efficiency of succimer used with calcium and ascorbic acid in the treatment of mild lead-poisoning. Environ Toxicol Pharmacol. 2011;31(1):137-42.
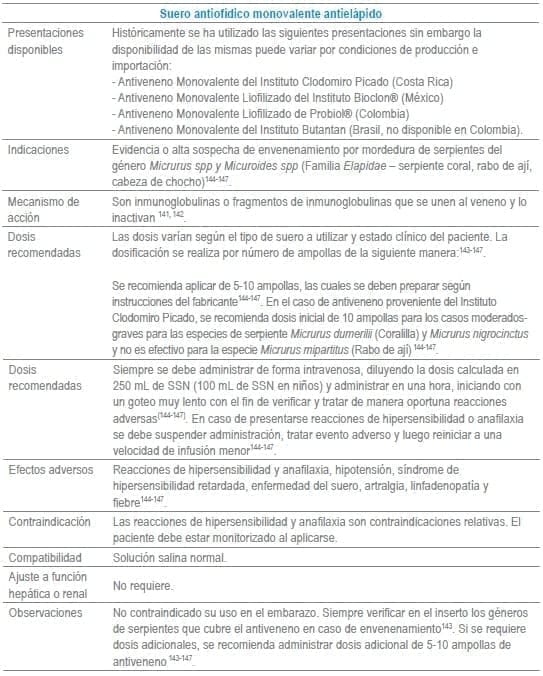
- 141. Hifumi T, Sakai A, Kondo Y, Yamamoto A, Morine N, Ato M, et al. Venomous snake bites: clinical diagnosis and treatment. J Intensive Care. 2015;3(1):16.
- 142. Maduwage K, Buckley NA, de Silva HJ, Lalloo DG, Isbister GK. Snake antivenom for snake venom induced consumption coagulopathy. Cochrane Database Syst Rev. 2015;6: Cd011428.
- 143. Lastra SM, Rodríguez A, La Rota JF. Anexo 3. Recomendaciones para el uso del suero antiofídico polivalente del Instituto Clodomiro Picado de Costa Rica en accidentes ofídicos producidos en el territorio colombiano. En: Protocolo de vigilancia epidemiológica. Accidente ofídico. Bogotá, Colombia: Instituto Nacional de Salud, Ministerio de Salud y Gobierno de República de Colombia; 2014. p. 26.
- 144. Lastra SM, Rodríguez A, La Rota JF. Anexo 3. Recomendaciones para el uso del suero antiofídico polivalente del Instituto Clodomiro Picado de Costa Rica en accidentes ofídicos producidos en el territorio colombiano. En: Protocolo de vigilancia epidemiológica. Accidente ofídico. Bogotá, Colombia: Instituto Nacional de Salud, Ministerio de Salud y Gobierno de República de Colombia; 2014. p. 25.
- 145. Instituto Clodomiro Picado. (2009). El envenenamiento por mordedura de serpiente en Centroamérica. Montes de Oca, San José, Costa rica: Universidad de Costa Rica.
- 146. Instituto Clodomiro Picado. (2009). Tratamiento del envenenamiento por mordedura de serpiente. San José: Instituto Clodomiro Picado.
- 147. Peña L, Martínez V (2010). Accidente ofídico elapídico. En: Peña L, Arroyave L, Aristizabal JJ, Gómez U. Toxicología clínica. Medellín: Corporacion para Investigaciones Biologicas. p 465-77.
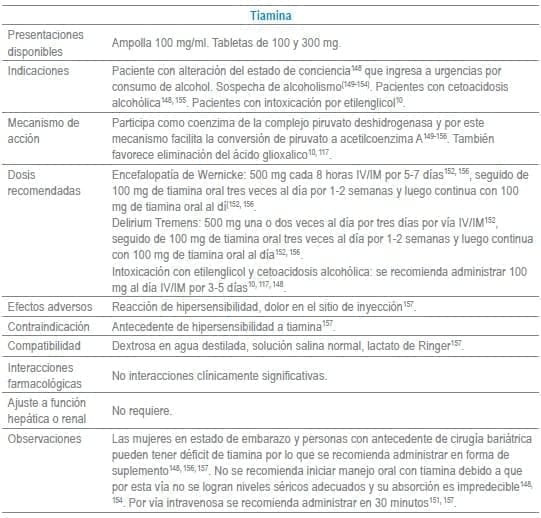
- 148. Hoffman RS. Antidotes in Depth. Thiamine Hydrochloride. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1094-100.
- 149. de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127(1):71-90.
- 150. Hillbom M, Saloheimo P, Fujioka S, Wszolek ZK, Juvela S, Leone MA. Diagnosis and management of Marchiafava-Bignami disease: a review of CT/MRI confirmed cases. J Neurol Neurosurg Psychiatry. 2014;85(2):168-73.
- 151. Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911-5.
- 152. Schuckit MA. Recognition and management of withdrawal delirium (delirium tremens). N Engl J Med. 2014;371(22):2109-13.
- 153. Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff Syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;7: Cd004033.
- 154. Ridley NJ, Draper B, Withall A. Alcohol-related dementia: an update of the evidence. Alzheimers Res Ther. 2013;5(1):3.
- 155. Scalzo SJ, Bowden SC, Ambrose ML, Whelan G, Cook MJ. Wernicke-Korsakoff syndrome not related to alcohol use: a systematic review. J Neurol Neurosurg Psychiatry. 2015;86(12):1362-8.
- 156. Kerns JC, Arundel C, Chawla LS. Thiamin deficiency in people with obesity. Adv Nutr. 2015;6(2):147-53.
- 157. Thiamine. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.
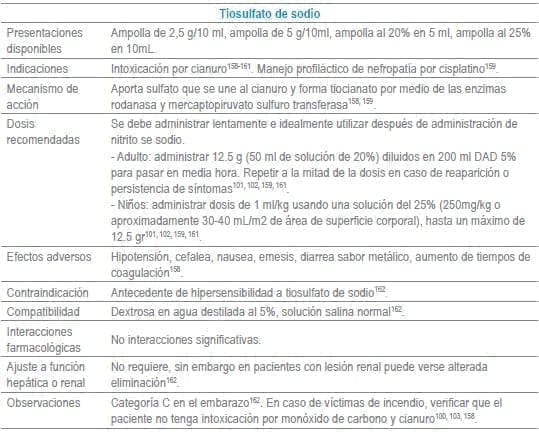
- 158. MacLennan L, Moiemen N. Management of cyanide toxicity in patients with burns. Burns. 2015;41(1):18-24.
- 159. Howland MA. Antidotes in Depth. Sodium Thiosulfate. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical; 2014. p. 1615-7.
- 160. Streitz MJ, Bebarta VS, Borys DJ, Morgan DL. Patterns of cyanide antidote use since regulatory approval of hydroxocobalamin in the United States. Am J Ther. 2014;21(4):244-9.
- 161. De La Calzada-Jeanlouie M, Coombs J, Shaukat N, Olsen D. Utility of sodium thiosulfate in acute cyanide toxicity. Ann Emerg Med. 2013;61(1):124-5.
- 162. Sodium thiosulfate: Drug information. En: UptoDate® [Internet]. Wolters Kluwer Health. 2016 [cited Abril 2016]. Disponible en: http://www.uptodate.com.ez.urosario.edu.co/.
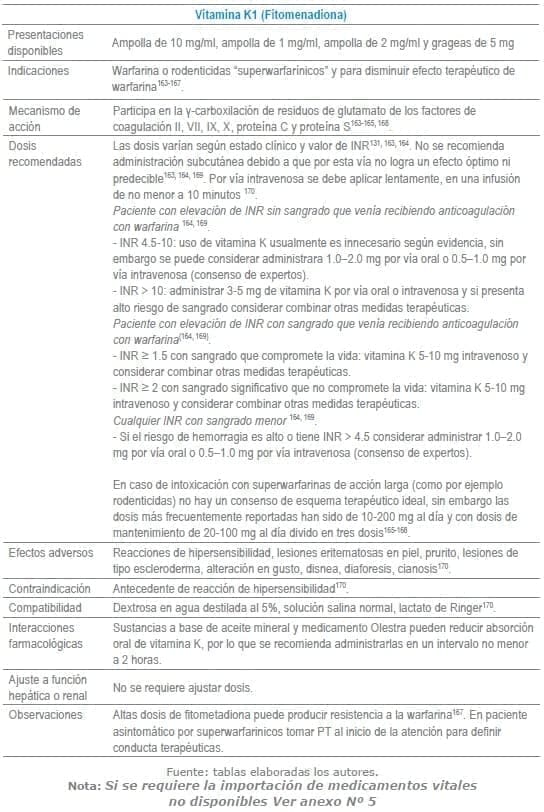
- 163. Matthews SS, Ringeisen AL, Wedro B. Intentional overdose of warfarin in an adult: anticoagulant reversal in the ED. Am J Emerg Med. 2014;32(9): 1150.e1-2.
- 164. Patriquin C, Crowther M. Treatment of warfarin-associated coagulopathy with vitamin K. Expert Rev Hematol. 2011;4(6):657-65; quiz 66-7.
- 165. Spahr JE, Maul JS, Rodgers GM. Superwarfarin poisoning: a report of two cases and review of the literature. Am J Hematol. 2007;82(7):656-60.
- 166. Bates D, Mintz M. Phytonadione therapy in a multiple-drug overdose involving warfarin. Pharmacotherapy. 2000;20(10):1208-15.
- 167. Howland MA. Antidotes in Depth. Vitamin K1. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s Toxicologic Emergencies. Tenth ed. New York, EU: McGraw-Hill Education/Medical 2014. p. 836-8.
- 168. King N, Tran MH. Long-Acting Anticoagulant Rodenticide (Superwarfarin) Poisoning: A Review of Its Historical Development, Epidemiology, and Clinical Management. Transfus Med Rev. 2015;29(4):250-8.
- 169. Tran HA, Chunilal SD, Harper PL, Tran H, Wood EM, Gallus AS. An update of consensus guidelines for warfarin reversal. Med J Aust. 2013;198(4):198-9.
- 170. Phytonadione. En: Micromedex® 1.0 (Healthcare Series) [Internet]. Truven Health Analytics Inc. 2016 [cited Abril 2016]. Disponible en: http://www.micromedexsolutions.com.







