Jorge Enrique Machado-Alba1, Santiago García-Betancurt1
Abstract
Objective: To learn physicians’ perspectives regarding the ethical considerations of receiving benefi ts from the pharmaceutical industry through its sales representatives.
Methods: Observational study, using phone interviews with general practitioners and specialists in Colombia, conducted between June 1 and July 15, 2014. The variables considered were age, sex, specialty, time as a practicing professional, place of work, level of care, city, and ethical perceptions of visits by representatives of the pharmaceutical industry and perceptions of gifts, restaurant invitations, conferences, trips, and money offered by the representatives. A multivariate analysis was per- formed.
Results: A total of 172 physicians were interviewed; of these. 68.0% were male with a mean age of 41±5.3 years, and they received a mean of 2.7 visits per week. A total of 90.1% of interviewees considered ethical to receive desktop items, 91.3% to receive drug samples, 87.2% to receive invitations to conferences, 60.5% to accept money for research projects, and 55.8% to receive money to hold conferences.
A total of 69.2% of the physicians believed that there tends to be a conflict of interest in the medical-pharmaceutical relationship, with statistically significant differences in physicians who work in public vs. private institutions (85.5% vs. 50.0%; p=0.002) and in general physicians vs. specialists (79.2% vs. 62.0%; p=0.04).
Discussion: The relationship between the pharmaceutical industry and physicians is composed of everyday interactions that can vary. The offer of different types of benefi ts is identifi ed as a source of potential confl icts of interest that may lead to ethical dilemmas in the practice of medicine.
Keywords: Ethics; Doctor Offi ce Visits; Confl ict of Interest; Pharmaceutical Industry; Pharma- coepidemiology; Colombia (source: MeSH) (Leer: Sobre la comprensión del trascurso del tiempo)
Percepción respecto a consideraciones éticas de los médicos del departamento de risaralda (colombia) derivadas de recibir beneficios de la industria farmacéutica
Resumen
Introducción: los médicos son visitados continuamente por representantes de los laboratorios farmacéuticos con fi nes promocionales.
Objetivo: conocer la percepción de los médicos res- pecto a las consideraciones éticas derivadas de recibir benefi cios o prebendas de la industria farmacéutica a través de sus representantes comerciales.
Métodos: estudio observacional, mediante entrevistas telefónicas a médicos generales y especialistas en Colombia, entre 1 de junio y 15 de julio de 2014. Se consideraron las variables edad, sexo, especialidad, tiempo de ejercicio profesional, lugar de trabajo, nivel de atención, ciudad, percepciones éticas sobre la visita de representantes de la industria farmacéutica, regalos, invitaciones a restaurantes, congresos, viajes, dinero. Se hizo análisis multivariado.
Resultados: se entrevistaron 172 médicos, el 68,0% eran hombres, edad promedio 41+5,3 años, recibían una media de 2,7 visitas semanales. El 90,1% de encuestados consideró ético recibir elementos de escritorio, 91,3% recibir muestras médicas, 87,2% recibir invitaciones a congresos, 60,5% aceptar dinero para proyectos de investigación, y 55,8% recibir dinero para realizar conferencias.
El 69,2% de los médicos cree que en la relación médico-industria farmacéutica suele existir un conflicto de intereses, observándose diferencias estadísticamente significativas entre los médicos que trabajan en instituciones públicas vs privadas (85,5% vs 50,0%; p=0,002) y entre médicos generales versus especialistas (79,2% vs 62,0%; p=0,04).
Discusión: la relación de la industria farmacéutica con los médicos representa una actividad cotidiana en la cual las interacciones pueden ser variadas, y el ofrecimiento de diferentes tipos de beneficios fue identificado dejando en evidencia la posibilidad de creación de potenciales conflictos de intereses y que dan pie a la generación de dilemas éticos al ejercer la profesión médica.
Palabras clave: Ética; Visita a Consultorio Médico; Conflicto de Intereses; Industria Farmacéutica; Farmacoepidemiología; Colombia (fuente: DeCS)
Introduction
Physicians are the target of promotional activities by pharmaceutical companies, investing a huge amount of money to promote their products using various sales techniques, including visits by sales representatives (1).
These visits can have a signifi cant impact on physicians’ preferences for certain drugs (2) and may generate potentially inappropriate prescrip- tions that contribute to increasing health costs and the lack of adherence to drugs’ approved guidelines due to the use of second-line drugs over recommended drugs (3).
Pharmaceutical companies are also blamed for the increase in the prescription of new medicines, including those that do not demonstrate any advantage when compared to traditional medicines with proven efficacy (4).
The marketing targeted at physicians includes verbal presentations that are typically accompanied by promotional brochures based on studies that occasionally are of poor methodological quality, and it has been found that up to 11% are inaccurate (3).
In the United States, approximately $11 billion are invested annually in strategies to increase pharmaceutical companies’ product sales, spending globally in 2012, $89.5 billion in sales and marketing expenses (5, 6). In addition, it has been reported that some physicians do not recognize the influence that these activities may have on their own decisions and the effect they may have on their clinical judgment, whereas they do recognize the influence they have on the prescriptions of other prescribers (1, 5, 7, 8, 9).
It has been shown that 50.8% of physicians think that there are no ethical problems in the relationship between the medical and the pharmaceutical industries; however, a conflict of interest is considered to exist when prescriptions are influenced by factors other than scientific information or when professional opinion on a patient’s care may be unduly influenced by an ulterior motive (10-12).
1 Grupo de Investigación en Farmacoepidemiología y Farmacovigilancia, Universidad Tecnológica de Pereira-Audifarma S.A. Pereira, Colombia.
There are no studies generating information on this issue in Colombia, furthermore there is a well-known positive attitude toward pharma representatives (8, 9, 13); in the other hand there are many recommendations included in different documents, plus laws and organizations such as “unbranded doctors”, that regulate medical behavior in relation to the pharmaceutical industry (14-18).
The aim of this paper is to learn about the perception of physicians regarding ethical caveats when receiving benefits or perks from the pharmaceutical industry through its sales representatives.
Methods
An observational study was conducted using telephone interviews to collect data from general physicians and specialists who work in the Department of Risaralda, Colombia, with a population of approximately 951,945 people. Of a total of 777 randomly selected physicians who work in the department, 278 were individually called and gave prior verbal informed consent to respond to a form with the questions to be considered. The answers were collected anonymously for each of the interviewees.
The data were collected between June 1 and July 15, 2014, and were tabulated in a database in Microsoft Excel 2007 by a duly trained physician.
Inclusion Criteria
Every randomly selected general practitioner or specialist who accepted participation and who worked in the Department of Risaralda. Physicians who simultaneously worked in that one and in another department, were also accepted.
Exclusion Criteria
• Physicians who did not agree to answer the survey.
The questionnaire used considered the following variables:
Sociodemographic
Age, sex, specialty, time as a practicing professional, place of work (public hospital, private clinics, private practice, or individual offices), level of care (primary, secondary, or tertiary care), and city where the doctor works, distinguishing between Pereira and other cities.
Ethical Perceptions
Regarding visits from pharmaceutical sales representatives and regarding gifts, invitations to restaurants, congresses, symposia, trips, and money. Ethical considerations regarding the abovementioned points and knowledge of the medical ethics law (Ley 23 del 1981).
The project was endorsed by the Bioethics Committee of the City of Pereira Technological University (Universidad Tecnológica de Pereira), under the category of investigation without risk, according to criteria established by the Declaration of Helsinki.
For the data analysis, the statistical software package SPSS 22,0 for Windows (IBM, Chicago IL, USA) was used. Frequencies and proportions were established. The X2 test was used to compare categorical variables.
Logistical regression models were applied using the ethical consideration according to the Medical Ethics law (yes/no) as a dependent variable and those that were significantly associated with the dependent variable in the bivariate analysis as covariables. The level of statistical significance was determined as P<0,05.
Results
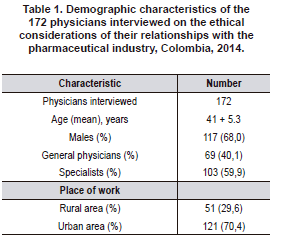
A total of 278 phone calls to physicians were made, of which 172 agreed to participate in the survey (61,8%). Of those interviewed, 68,0% were male, and the mean age was 41 years (range: 23-73 years). The population characteristics are shown in Table 1.
With regard to the number of visits from pharmaceutical sales representatives, those interviewed reported a mean of 2,7 visits per week (range: 0-60). Regarding the items received during visits, 95,9% received medical samples; 95,3% desktop items; 78,5% invitations to lectures, symposia, and/or conferences; 48,8% invitations to meals; 23,3% trips to attend lectures, symposia, and/ or conferences; 8,7% money to hold conferences; 4,1% money to conduct research projects; and 0,6% trips so that family can attend lectures, symposia, and/or conferences.
A total of 57,6% (n=99) of interviewees offered the opinion that pharmaceutical companies’ strategies for the promotion of medications influence medical prescriptions in general, and 22,1% (n=38) admitted that they influence their own prescribing. It was found that 54,1% (n=93) agree with the strategies employed by pharmaceutical companies for the promotion of their products (Figure 1). Table 2 shows the percentage of physicians who considered ethical to receive various benefits from the pharmaceutical industry.


Of the total interviewees, 48,3% were aware of the existence of a law regulating medical ethics, and 50,0% knew that this law prohibits the receipt of any type of commercial benefit by laboratories, eye care and orthopedic centers, and other organizations selling prescription items.
Bivariate Analysis
There were statistically significant differences among physicians who found ethical to receive drug samples while working in public hospitals against those working in private ones (83,9% vs. 95,5%; p=0,01), among physicians in private practice or not affiliated with health institutions and other physicians (98,4% vs. 87,4%; p=0,02), or physicians who worked in the city of Pereira and those who worked in other cities (97,0% vs. 83,1%; p=0,005).
In regard to ethical perception about accepting invitations to meals from pharmaceutical companies, there also were statistically significant differences for those working in public hospitals and those who worked in another places (29,0% vs. 67,3%; p<0,001), and for the above aforementioned second group of physicians (73,8% vs. 42,3%; p<0,001); for physicians who worked in tertiary care and other type of doctors (40,3% vs. 60,9%; p=0,03), and for physicians who worked in Pereira and those who worked in other cities (62,4% vs. 39,4%; p=0,008).
Concerning acceptance of invitations to attend lectures, symposia, and/or conferences, 87,2% of physicians found it ethical. Statistically significant differences were found for doctors working in Pereira and those in other cities (91,1% vs. 81,7%; p=0.01).
Regarding ethicality of paid trips to attend lectures, symposia, and/or conferences, there were statistical differences for public hospital doctors accepting this behavior against those who worked in other settings (40,3% vs. 64,5%; p=0,006), those in primary care level and other physicians (19,0% vs. 60,9%; p=0,001), those who worked in the city of Pereira and those in other cities (64,4% vs. 43,7%; p=0,005), physicians who worked in the city of Santa Rosa and those in other cities (27,8% vs. 59,1%; p=0,01); there were also differences between the groups of general practitioners and specialists (40,3% vs. 67,0%; p=0,002).
No significant differences were found for the questions of whether is ethical to receive the following: desktop items; paid travel expenses for accompanying family members to lectures, symposia, and/or conferences; and honorary fees to carry out clinical trials.
Finally, 69,2% of the physicians surveyed believed that there tends to be a conflict of interest in the medical-pharmaceutical relationship, observing statistically significant differences between those physicians who worked in public and those who worked in private institutions (85,5% vs. 50,0%; p=0,002), between physicians in private practice and other physicians (49,2% vs. 80,2%; p<0.001), and between general physicians and specialists (79,2% vs. 62,0%; p=0,04).
Discussion
This study allowed us to learn about the ethical considerations taken into account by physicians when interacting with the pharmaceutical industry. The rate of response of the medical population to this survey was higher than that found in similar studies (61,8% vs. 28,1%, 25,9%, and 25,9%) (1,12,19), although one reported a higher response rate (81,0%) (20).
The mean number of visits found in this study was 2,7 per week; this figure contrasts with studies conducted in Spain, the Netherlands, Germany and Denmark, which show variable weekly means of 10,2, 1,4, 1 and 0,9, respectively (1, 8,21,22). Meanwhile, in 2009, the mean number of visits for physicians in the United States was similar to that found in this study: 2,3 (23).
When data on different benefits offered by the pharmaceutical industry found in this study are compared with that from similar studies, it is possible to see a variable pharma-doctors relationship. The percentage of professionals who receive drug samples is typically greater than 80,0% (20,24,25), also evidenced in a recent systematic review as one of the most common gifts, as well as dinner invitations, (9); a study conducted by Birkhahn et al. in 2008 shows that only 41,0% reported accepting samples (26).
In this study, the percentage of physicians who receive desktop items (95,3%) contrasts with results in other study, in which only 39,0% reportedly accepted such items (20).
For the variables corresponding to invitations to lectures, symposia, and/or conferences; receiving invitations to meals; and receiving trips to attend lectures, symposia, and/or conferences, the percentages of acceptance are different from those found in this study (4,0% vs 80,0%) (20,24,25,27).
Accepting money for holding conferences is analyzed in one United States study, reporting a higher percentage than in our study (20,0% vs. 8,7%) (26).
Percentage of those who accept the different types of benefits in other studies versus the present one, contrast with the recommendations of different associations, organizations, and authors who express that is impossible to have impartial judgment when there are interests at play and that the acceptance of a gift of whatever size imposes a strong payback obligation, thus affecting doctor´s objectivity (28-31).
No data was found in the literature regarding physicians’ acceptance of money to conduct clinical trials or their acceptance of travel support for their family could join, while attending lectures, symposia, and/or conferences.
Perception of pharmaceutical companies’ strategies to influence the writing of prescriptions in general and on the physicians’ own prescriptions, were similar both for this study and another ones; there is evidence that physicians recognize that other prescribers may be influenced, not being the case for themselves (1,9,20,24).
The greater the financial value of the incentive, the lesser the ethics of the acceptance, as shown in this paper; similar findings are presented in two studies conducted in Spain that evaluate the ethics in the relationship between doctors and pharmaceutical sales representatives (12,19).
Recommendations made by the American Medical Association propose gifts acceptance when they are of minimal value and/or when they benefit the patient directly, i.e. patient’s education (31); in the other hand, local regulations forbids receipt of any type of benefit by commercial laboratories among others (16,17).
Efforts through different legal mechanisms and recommendations should address public awareness of any type of benefit received (14,15,18), which in a way is also contrary to what is expressed in local regulation.
When evaluating the data on the ethical considerations of accepting trips to attend lectures, symposia, and/or conferences, this study found statistically significant differences between general practitioners and specialists (40,3% vs. 67,0%); similar data was found in studies conducted in Spain and the United States, where dermatologists and surgeons showed statistically significant differences in accepting this type of benefit (20, 25).
This situation may be explained by the fact that specialists may be a group of greater interest to pharmaceutical companies due to their potential prescription of more expensive medicines, not typically done by generalists; which is also evidenced in a recent systematic review showing a greater acceptance of medical samples by specialists (9).
Similarly, observing data from the bivariate analysis, we show that physicians who work in the public sector have the lowest percentage considering ethical to receive different types of benefits; these physicians also believe, in a statistically significant manner, that there is a conflict of interest with the pharmaceutical industry. This group adheres somehow to recommendations established by different global associations concerning the relationship between physicians and the pharmaceutical industry (10, 28,30,31).
This study has limitations. First, although the rate of response is higher than that of other similar studies, obtaining a 100% response would offer a better overview on this issue.
Second, the surveyed population is a representative sample of the Department of Risaralda, not of the entire country; however, this study provides information on a controversial issue, difficult to address for the medical community, given the absence of similar studies, and collects information from professionals who work in both private and public sectors. Finally, answers to the questionnaire may not have been entirely honest, given that the topic is controversial.
We conclude that the relationship between the pharmaceutical industry and physicians have everyday interactions that can vary. Almost all physicians interviewed report frequent visits from representatives and a relationship in which the offer of different types of benefits is identified as a source of potential conflict of interest.
Although these conflicts are not visible to the entire physician´s population, they remain present and can lead to ethical dilemmas in the practice of medicine.
Therefore, it is necessary to conduct further studies that evaluate the perception of this relationship at the national level, also including medical students who are targeted by pharmaceutical representatives. It is necessary for national medical organizations to deliver clear recommendations on how to approach this type of interaction.
Sources of funding
This study received funding from the Universidad Tecnológica de Pereira, and Audifarma S.A.
Declaration of conflict of interest
The authors declare that they have no conflict of interest.
Contributor´s statements
Jorge Enrique Machado-Alba participated in the drafting, data collection, data analysis, description of results, discussion, critical revision of the article, and evaluation of the final version of the manuscript. Santiago Garcia participated in the drafting, data collection, data analysis, description of results and discussion.
References
1. Garcés Redondo G, Colán Colán C, Sánchez Oro¬pesa A, Gómez Suanes G, Canchig Pilicita F, López de Castro F. Opinión sobre la Visita Médica de los Médicos de Atención Primaria de Toledo. Rev Clin Med Fam. 2010; 3:5-9.
2. Søndergaard J, Vach K, Kragstrup J, Andersen M. Impact of pharmaceutical representative visits on GPs’ drug preferences. Fam Pract. 2009; 26:204-9. doi: 10.1093/fampra/cmp010.
3. Cardarelli R, Licciardone JC, Taylor LG. A cross-sectional evidence-based review of pharmaceutical promotional marketing brochures and their underlying studies: is what they tell us important and true? BMC Fam Pract. 2006; 7:13.
4. Blumenthal D. Doctors and drug companies. N Engl J Med. 2004; 351:1885-90.
5. Wazana A. Physicians and the pharmaceutical indus¬try: is a gift ever just a gift? JAMA. 2000; 283:373-80.
6. Chressanthis GA, Sfekas A, Khedkar P, Jain N, Poddar P. Determinants of pharmaceutical sales representative access limits to physicians. J Med Marketing. 2014;14(4):220-43.
7. Coyle SL; Ethics and Human Rights Committee, American College of Physicians-American Society of Internal Medicine. Physician-industry relations. Part 1: individual physicians. Ann Intern Med. 2002; 136:396-402.
8. Lieb K, Scheurich A. Contact between Doctors and the Pharmaceutical Industry, Their Perceptions, and the Effects on Prescribing Habits. PloS one. 2014;9(10).
9. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical indus¬try generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open 2017;7:e016408. doi:10.1136/ bmjopen-2017-016408
10. Asociación Médica Mundial. Declaración de la AMM sobre conflictos de intereses. Nueva Delhi. 2009: 1-6. [Fecha de consulta: Mayo 7 del 2015]. Disponible en: http://www.scp.com.co/simposio/descargas/AMM.pdf
11. Salas SP, Osorio F M, Vial C P, Rehbein V AM, Salas A C, Beca I JP. (Conflicts of interest in clinical practice. Ethical analysis of some relationships with the pharmaceutical industry). Rev Med Chile. 2006; 134:1576-82.
12. Grup d’Ética. Societat Catalana de Medicina Familiar y Comunitaria. La ética en la relación con la industria farmacéutica. Encuesta de opinión a médicos de familia en Cataluña. Aten Primaria. 2004; 34:6-12.
Lotfi T, Morsi RZ, Rajabbik MH, Alkhaled L, Kahale L, Nass H, et al. Knowledge, beliefs and attitudes
of physicians in low and middle-income countries regarding interacting with pharmaceutical compa¬nies: a systematic review. BMC Health Services Research. 2016;16
14. Concejo Nacional de Política Económica y Social. Política Farmacéutica Nacional. 2012: 1-49. [Fecha de consulta: Octubre 23 del 2017]. Disponible en: https://www.minsalud.gov.co/Documentos%20y%20 Publicaciones/Politica%20Farmac%C3%A9utica%20 Nacional.pdf
15. Ministerio de salud y protección social. Decálogo de medidas prioritarias de transparencia e integridad para la regulación de precios de medicamentos y la definición del plan de beneficios en Colombia. 2015: 1-8. [Fecha de consulta: Octubre 23 del 2017]. Disponible en: https://www.minsalud.gov.co/sites/ rid/Lists/BibliotecaDigital/RIDE/VS/MET/decalogo-transparencia-integridad-sectorsalud.pdf
16. Ley estatutaria de salud 1751 del 16 de Febrero de 2015. (Diario Oficial No. 49.427 de 16 de febrero de 2015)
17. Ley 1474 del 2011 del 12 de Julio de 2011, de me¬canismos de prevención, investigación y sanción de actos de corrupción y la efectividad del control de la gestión pública. (Diario Oficial No. 48.128 de 12 de julio de 2011)
18. Ministerio de salud y protección social. Proyecto de resolución por el cual se adoptan medidas para garantizar la trasparencia en las relaciones de los agentes del sector salud y la industria farmacéutica y de tecnologías en salud. 2014: 1-8. [Fecha de con¬sulta: Octubre 23 del 2017]. Disponible en: https:// www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/ RIDE/VS/MET/Proyecto-Resolucion-regulacion-sector-farmaceutico-profsalud.pdf
19. Galán Herrera, M.T. Delgado Marroquín, R. Altisent Trota. Análisis de la relación entre el médico de atención primaria y la industria farmacéutica. Aten Primaria. 2004; 34(5):231-247.
20. Castreana L, Mejía R, Aznar M. Actitud de los médicos frente a las prácticas de promoción de la industria farmacéutica. Medicina (B Aires). 2005; 65:247-51.
21. Muijrers PE, Grol RP, Sijbrandij J, Janknegt R, Knottnerus JA. Differences in prescribing between GPs: impact of the cooperation with pharmacists and impact of visits from pharmaceutical industry representatives. Fam Pract. 2005; 22:624-30.
22. Schramm J, Andersen M, Vach K, Kragstrup J, Kamp¬mann JP, Søndergaard J. Promotional methods used by representatives of drug companies: a prospective survey in general practice. Scand J Prim Health Care. 2007; 25:93-7.
23. Fischer MA, Keough ME, Baril JL, Saccoccio L, Mazor KM, Ladd E, et al. Prescribers and pharmaceutical representatives: why are we still meeting? J Gen Intern Med. 2009; 24:795-801.
24. De Ferrari A, Gentille C, Davalos L, Huayanay L, Malaga G. Attitudes and relationship between phy-sicians and the pharmaceutical industry in a public general hospital in Lima, Peru. PLoS One. 2014; 9(6):e100114.
25. Korenstein D, Keyhani S, Ross JS. Physician attitudes toward industry: a view across the specialties. Arch Surg. 2010; 145:570-7.
26. Birkhahn RH, Blomkalns AL, Klausner HA, Nowak RM, Raja AS, Summers RL, et al. Academic emer-gency medicine faculty and industry relationships. Acad Emerg Med. 2008; 15:819-24.
27. World Medical Association. WMA Statement con¬cerning the relationship between physicians and commercial enterprises. Nueva Delhi. 2009. 1-4. (Fecha de consulta: Mayo 7 del 2015). Disponible en: http://advamed.org/res.download/78.
28. Moskop JC, Iserson KV, Aswegan AL, Larkin GL, Schears RM. Gifts to physicians from industry: the debate evolves. Ann Emerg Med. 2012; 59:89-97.
29. Asociación Médica Mundial. Declaración de Lisboa de la AMM sobre los derechos del paciente. Santiago de Chile. 2005. 1-4.
30. Schetky DH. Conflicts of interest between physicians and the pharmaceutical industry and special interest groups. Child Adolesc Psychiatr Clin N Am. 2008; 17(1):113-25.
31. American Medical Association. Code of medical ethics. Opinion 8.061-Gifts to Physicians from In¬dustry. (Fecha de consulta: Mayo 7 del 2015). Disponible en: http://www.ama-assn.org/ama/pub/ physician-resources/medical-ethics/code-medical-ethics/opinion8061.page
Received: August 10, 2017
Approved: October 30, 2017
E-mail:
Jorge Enrique Machado Alba
machado@utp.edu.co

