Association between Chronic Lymphocityc Thyroiditis and Papillary Thyroid Carcinoma: Systematic review and meta-analysis of studies on surgical specimens
Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides: Revisión sistemática y metaanálisis de estudios en especímenes quirúrgicos
Abstract
Introduction. Inconsistent results exist in the literature regarding the hypothesis statement suggesting an increased likelihood of documenting papillary thyroid carcinoma (PTC) in surgical specimens with changes compatible with chronic lymphocytic thyroiditis. Existing meta-analyses have included studies that are not methodologically comparable and do not propose clear sources of bias, thus, this is justification for the present meta-analysis.
Methods. A literature search in PubMed and Embase was performed from January 1, 1950 to December 31, 2020. Retrospective studies comparing the prevalence of papillary thyroid carcinoma in specimens with and without chronic lymphocytic thyroiditis changes were obtained. The collected evidence was statistically analyzed.
Results. A total of 22 articles were included. The study population consisted of 63,548 surgical specimens. The pooled OR, based on the studies, was 1.81 (95% CI: 1.51-2.21). There was heterogeneity between the distribution of prevalence ratios and opportunity ratios across studies (I²= 91%; p>0.00001). The funnel plot shape of the studies included in the analysis appears to be symmetrical, indicating the absence of bias attributable to small studies.
Conclusions. The current literature suggests that there is an increased risk of documenting papillary thyroid carcinoma in surgical specimens in which chronic lymphocytic thyroiditis-compatible changes are observed; however, there are sources of bias that will not be possible to control for in retrospective studies, so we recommend studying the hypothesis suggesting an increased likelihood of diagnosing PTC in specimens with chronic lymphocytic thyroiditis-compatible changes using prospective methodologies.
Keywords: papillary thyroid carcinoma; chronic lymphocytic thyroiditis; surgical specimens; retrospective study; systematic review; meta-analysis.
Resumen
Introducción. Existen resultados inconsistentes con relación al planteamiento de la hipótesis que sugiere una mayor probabilidad de documentar un carcinoma papilar de tiroides en especímenes quirúrgicos con cambios compatibles con tiroiditis linfocítica crónica. En los metaanálisis se han incluido estudios no comparables metodológicamente y no se proponen claras fuentes de sesgo, justificación para la realización del presente estudio.
Métodos. Se realizó una búsqueda bibliográfica en PubMed y Embase. Fueron obtenidos estudios retrospectivos donde se comparaba la prevalencia de carcinoma papilar de tiroides en especímenes con y sin cambios por tiroiditis linfocítica crónica. La evidencia recolectada fue sintetizada estadísticamente.
Resultados. Un total de 22 artículos fueron incluidos. La población estuvo conformada por 63.548 especímenes. El OR combinado fue 1,81 (IC95%: 1,51-2,21). Hubo heterogeneidad en la distribución de las razones de oportunidad entre los estudios (I2= 91 %; p>0,00001). La forma del gráfico en embudo de los estudios incluidos en el análisis parece estar simétrica, lo que indica la ausencia del sesgo atribuible a los estudios pequeños.
Conclusiones. La literatura actual sugiere que existe un mayor riesgo de documentar un carcinoma papilar de tiroides en especímenes quirúrgicos en los que se observan cambios compatibles con tiroiditis linfocítica crónica; sin embargo, existen fuentes de sesgo que no será posible controlar en estudios retrospectivos, por lo que recomendamos estudiar la hipótesis que sugiere una mayor probabilidad de diagnosticar un carcinoma papilar de tiroides en especímenes con cambios compatibles con tiroiditis linfocítica crónica mediante metodologías prospectivas.
Palabras clave: carcinoma papilar de tiroides; tiroiditis linfocítica crónica: especímenes quirúrgicos; estudio retrospectivo; revisión sistemática; metaanálisis.
Introduction – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
The concept of papillary thyroid carcinoma (PTC) encompasses a group of epithelial neoplasms of malignant biological behavior that present evidence of histogenesis from the follicular epithelium of the thyroid gland and a set of distinctive nuclear features1. Regarding its incidence, when analyzing the statistics reported in the last 20 years by the American Cancer Society, there are two epidemiological phenomena.
First, since 2000 and up to 2016, a 350% increase in the diagnosis of PTC was observed2; this is explained by the increase in the diagnosis of papillary microcarcinomas, a phenomenon attributable to the implementation of the Thyroid Imaging Reporting and Data System and the Bethesda System for Reporting Thyroid Cytopathology3. Second, since 2016 until now, a 39% decrease in the diagnosis of PTC was observed, from 3.8% to 2.3%4.
One of the hypotheses that seems to explain this phenomenon is attributed to the recategorization of thyroid neoplasms in 2016 by the World Health Organization1, and particularly to the redefinition of “encapsulated, non-infiltrating follicular neoplasm with nuclear changes similar to papillary carcinoma”, as a neoplasm with benign biological behavior5.
Hashimoto disease (HD) corresponds to a spectrum of autoimmune thyroid disease, in which there are positive serum titers for antibodies against specific thyroid proteins, always associated with clinical evidence of thyroid disease6.
It is considered the most frequent cause of hypothyroidism and hyperthyroidism in areas of the world with adequate and exaggerated iodine intake, respectively7.
Its age of highest incidence is between the fifth and sixth decade of life, and it is considered a female disease, with a female:- male ratio of at least 7:18.
HD can be associated with microscopic chronic lymphocytic thyroiditis (CLT) –compatible changes, consisting of diffuse replacement of the thyroid parenchyma by a mononuclear infiltrate, formed by lymphocytes and plasmacytes grouped in lymphoid follicles with germinal centers, associated with a variable degree of follicular atrophy, squamous and oncocytic metaplasia9.
The first publication found in the literature reporting the frequency of PTC in specimens with CLT was published in 1952 by Lindsay et al:
Who documented a prevalence of 21%10. However, the hypothesis of a higher prevalence of PTC in specimens with CLT was first evaluated analytically by Dailey et al in 195511.
Three methodological approaches suggesting a possible association between CLT and PTC, based on the retrospective study of surgical specimens, can be found in the literature. Two of these approaches use a descriptive approach and cross-sectional measurement to determine the prevalence of PTC in specimens with CLT, or vice versa.
A limitation of these approaches is the absence of comparison groups, typical of the analytical approach, which prevents the implementation of association statistics. However, when this hypothesis is evaluated with a retrospective, cross-sectional and analytical methodology, like the one proposed by Dailey et al in which the prevalence of PTC in specimens with and without CLT-compatible changes is compared, an association seems to be suggested.
Contradictorily, when this hypothesis is evaluated by analytical, prospective and cross-sectional methodological proposals, where ultrasound and cytological evaluation of nodular lesions is performed in patients with and without HD, independently of the presence or absence of changes compatible with CLT, in order to document the presence or absence of a PTC, it has not been possible to confirm the hypothesis suggested by retrospective studies, and controversially, suggest that there is no real increase in risk12-14 .
There are five meta-analysis studies in the literature in which this hypothesis is evaluated.
The results obtained from authors such as Singh et al15 with 11 studies up to 1999, Lee et al16 with 35 studies up to 2013, Lai et al17, and Resende et al18 with 47 studies up to 2017, suggest that there is a higher prevalence of PTC in specimens with CLT-compatible changes.
However, Jankovic et al19 with 35 studies up to 2013, in addition to suggesting such an association in their results, propose that these may be masked by a probable selection bias.
We consider that 14 of the 47 articles evaluated in these studies do not meet methodological requirements that allow their comparison: one of the articles corresponded to a cohort study20, three articles did not have surgical specimens as study objects, these consisted of prospective studies that performed ultrasound and cytological evaluation of patients with and without a diagnosis of HD and documented the suspicion or presumptive diagnosis of PTC in both groups21-23.
Finally, ten articles, although they did have surgical specimens as objects of study, did not have a comparison group, and therefore, it was not possible to compare the prevalence of PTC in specimens with and without changes compatible with CLT24-33.
Because there are inconsistent results regarding the association between CLT and PTC, in addition to the fact that existing meta-analyses have included methodologically non-comparable studies without proposing clear sources of selection bias and that many other related studies have been published in recent years, we performed a comprehensive meta-analysis to investigate the possible associations of CLT and PTC.
Methods – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
Search strategy and inclusion criteria
The systematic review process was performed according to the parameters established in The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses34, the systematization was performed by implementing the Review Manager 5.3 software and was summarized according to the flowchart proposed by the PRISMA Statement35, the proposed methodology was included in the International Prospective Register of Systematic Reviews (PROSPERO) database of the Center for Reviews and Dissemination of the National Institute for Health Research under ID CRD42020168562.
The literature search was performed in the databases Excerpta Medica dataBASE – Embase – of the Elsevier Publishing House and Medline – PubMed Central – of the US National Library of Medicine, the search period was established from January 1, 1950 to December 31, 2020, the search criteria were defined by the following group of descriptors: (“Hashimoto Disease” OR “Hashimoto Thyroiditis” OR “Hashimoto” OR “Thyroiditis” OR “Chronic Lymphocytic Thyroiditis” OR “Lymphocytic Thyroiditis” OR “Chronic Thyroiditis”) AND (“Papillary Thyroid Carcinoma” OR “Papillary Carcinoma” OR “Thyroid Carcinoma”).
The study selection process was carried out in five stages, based on the following definitions:
A study was considered as one that addressed the central theme when the study of the association of PTC in patients with CLT or HD was implicit in its title or in its structured abstract, the inclusion criteria were as follows: 1.
The object of study had to be defined as a surgical specimen; 2, the methodology of the original articles had to be retrospective, cross-sectional and analytical; 3, the diagnosis of CLT had to be made with histological parameters, with or without confirmation by anti-thyroid antibodies; 4, the diagnosis of PTC should have been made according to temporal parameters and defined by the International Classification of Endocrine Tumors proposed by the World Health Organization; and 5, the main purpose of the study should have been, therefore to compare the prevalence of PTC in two groups of surgical specimens, categorized according to the presence or absence of histological changes compatible with CLT.
Exclusion criteria
Once the concept of a study addressing the central theme and the inclusion criteria were defined, exclusion criteria were applied in the following phases.
During phase I, duplicate studies were excluded; during phase II, studies that did not address the central theme were excluded; in phase III, articles were excluded, although they addressed the central theme, were not original studies and, if they were, were not retrospective, cross-sectional and analytical in methodology. In addition, during this phase, articles whose complete body text was not found were excluded.
When there was no consensus on the application of a criteria, a researcher defined the exclusion or inclusion of that article during this phase. During phase IV, after the review of the complete body of the articles, those in which the total of the surgical specimens available during the study period were not included in the study population to be analyzed were excluded, since this would create an important selection bias.
Data collection process
Data collection was carried out independently by two researchers and tabulated in a pilot form. Subsequently, it was unified in a database by a third researcher, and in cases where there was a discrepancy, the data from the corresponding article were extracted by the latter.
From each article, the number of events was collected as follows: Group 1, case with CLT-compatible changes in surgical specimen, in which a PTC was documented; Group 2, case without CLT-compatible changes in surgical specimen, in which a PTC was documented; Group 3, case with CLT-compatible changes in surgical specimen, in which a PTC was not documented; and Group 4, case without CLT-compatible changes in surgical specimen, in which a PTC was not documented. The variables were tabulated using Excel 2020 ® software.
Statistical analysis
For the statistical analysis, the Forest diagrams, the calculation of statistic heterogeneity and evaluation of the bias inherent to meta-analysis studies, the Review Manager ® 5.3 and SPSS ® 25.0 statistical packages were used.
For the descriptive analysis, the frequency of specimens with findings compatible with CLT, the frequency of specimens with findings compatible with PTC and the frequency of specimens with simultaneous diagnosis of PTC and CLT were calculated, for the bivariate inferential analysis, contingency tables were constructed, and the prevalence ratio (PR) and odds ratio (OR) were calculated for the diagnosis of PTC in specimens with and without findings compatible with CLT in each of the studies, the statistical significance of this association was calculated using Pearson’s Chi-square test, P values less than 0.05 were considered statistically significant.
Inferential analysis and statistical heterogeneity
Statistical heterogeneity was calculated using a random effects model, justified by the fact that it is not possible to rule out the presence of clinical heterogeneity in the studies. OR was used as the effect estimator. To evaluate statistical heterogeneity, the Cochran-Mantel-Haenszel or Q Test statistic was used, and the presence of heterogeneity was defined with a value of p<0.1.
The I2 statistic was used to estimate the degree of variability in the estimate of the overall effect that is attributable to the heterogeneity of the studies in the analysis. The information and results corresponding to the stratified inferential analysis and the assessment of statistical heterogeneity were summarized using a Forest Diagram. Finally, funnel plots were used to assess the presence of bias attributable to the weight of small studies. To measure this, symmetry was visually assessed using a funnel plot.
Results – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
After exclusion of duplicate articles, the literature search strategy identified 3597 potentially eligible articles.
A total of 36 articles met the inclusion criteria, that is, their main purpose was to compare the prevalence of PTC in two groups of surgical specimens, categorized according to the presence or absence of histological changes compatible with CLT; however, 11 articles were excluded in which, after a thorough review of their methodology, it was possible to conclude that not all the specimens available during the study period were included in the statistical analysis36-46; on the other hand, in three of the articles47-49, despite the inclusion of all the specimens available during the study period, after the application of the Chi-square test, the count of expected phenomena was lower than the minimum, which suggested that the sample assessed could be insufficient to perform an inferential analysis, which is why they were excluded.
Finally, 22 articles50-71 were included in which, in addition to meeting the inclusion criteria, it was possible to perform an inferential analysis, with the lowest possible source of selection bias (Figure 1).
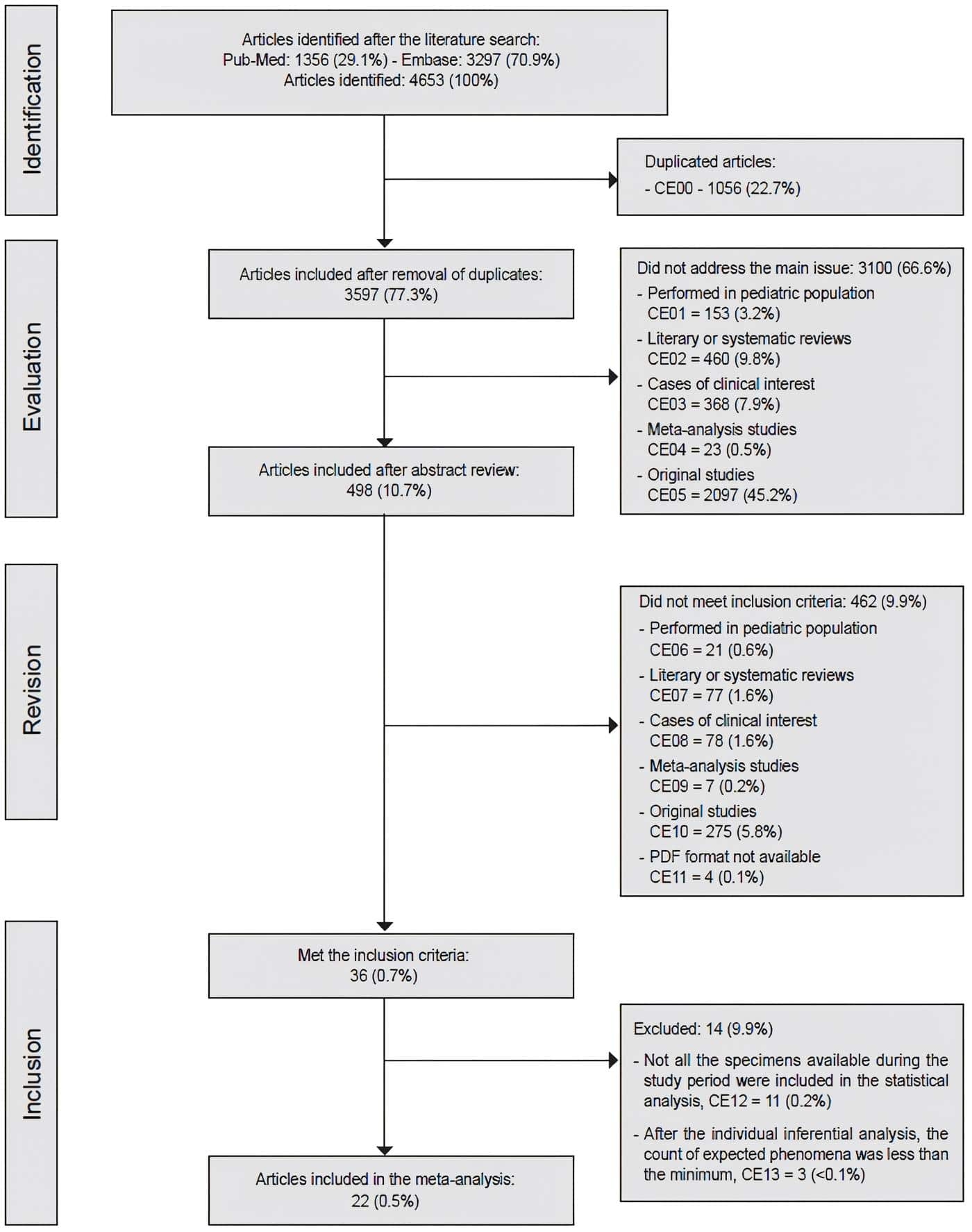
Figure 1. Sequence of exclusion and inclusion of articles in the study. Source: Prepared by the authors.
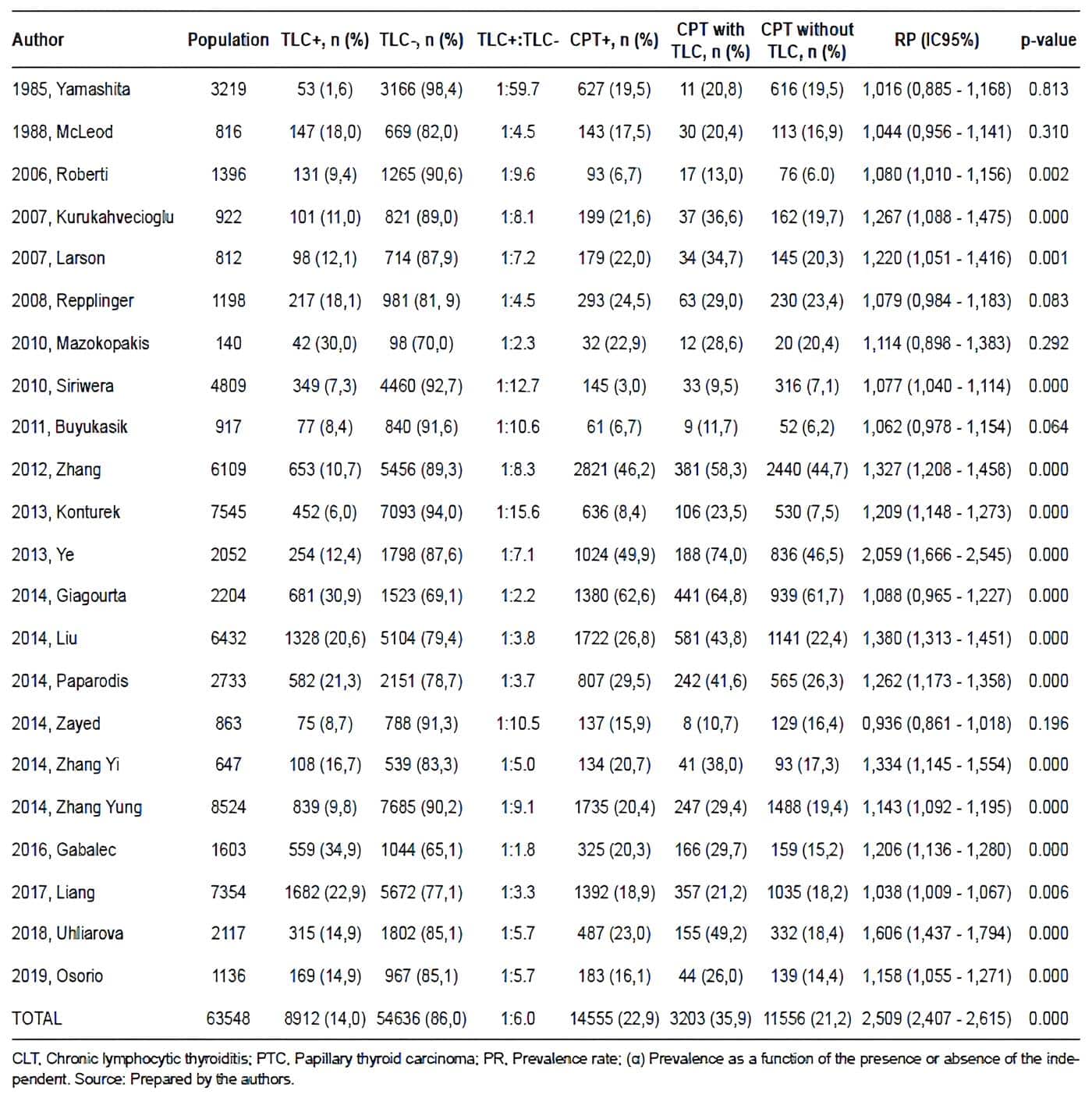
The study population consisted of 63,548 surgical specimens. The average prevalence of CLT and PTC in surgical specimens was 15.5% and 22.9%, respectively, the average prevalence of concurrence between PTC and CLT in surgical specimens was 32.5%. Table 1 summarizes the results of the descriptive and inferential statistical analyses performed from the data extraction of each of the articles.
The pooled OR, based on the studies, was 1.81 (95% CI: 1.51-2.21). However, there was significant heterogeneity between the distribution of PR and OR across studies (I2= 91%; p>0.00001) (Figure 2). The shape of the funnel plot of the studies included in the analysis appears to be symmetrical, indicating the absence of bias attributable to small studies (Figure 3).
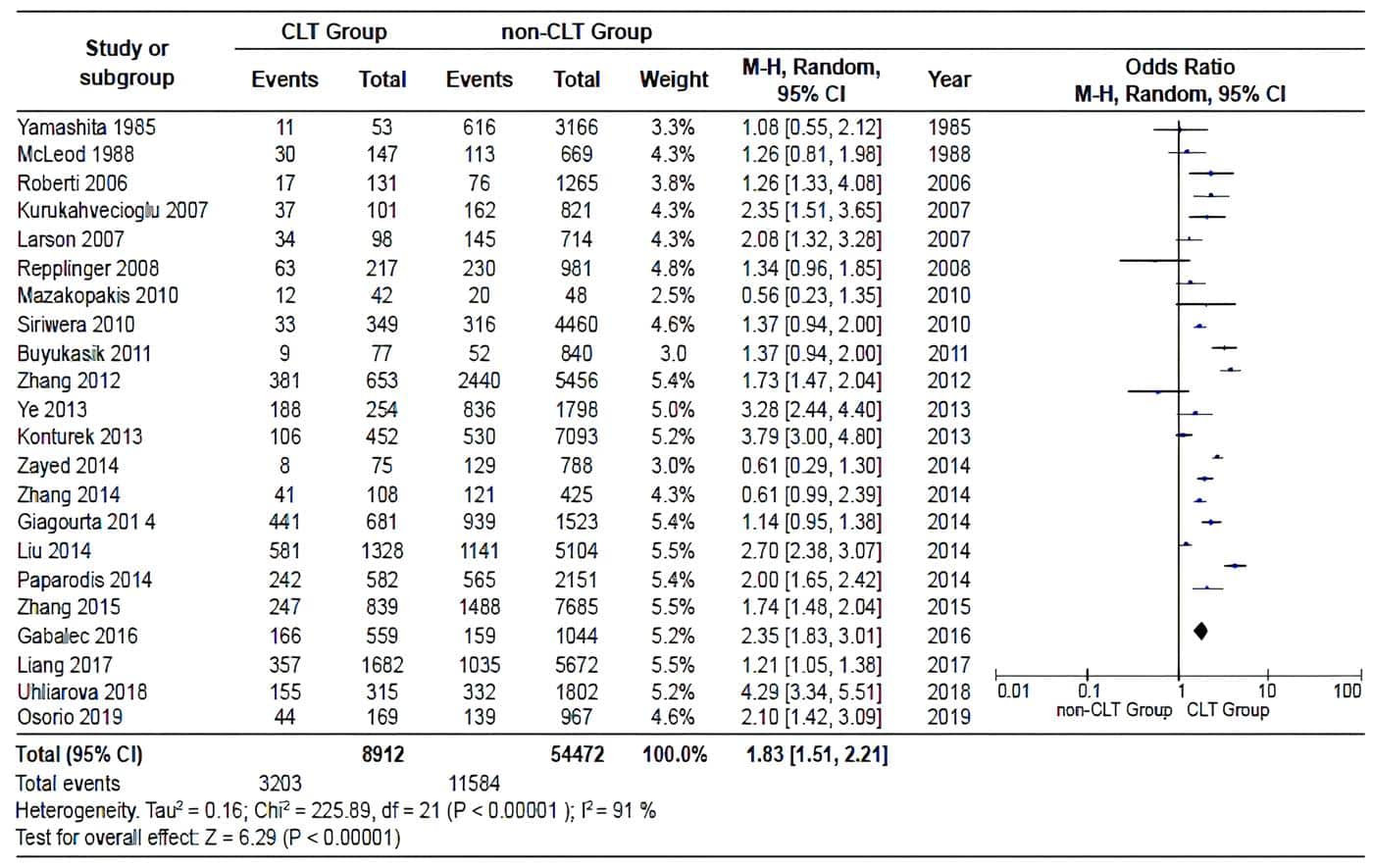
Figure 2. Pooled estimate of the risk of diagnosing a PTC in specimens with and without CLT,
according to the data extracted in each of the included studies. Source:
Prepared by the authors using Review Manager ® 5.3 software.

Figure 3. Funnel plot to estimate the bias attributable to small studies, for studies comparing
the risk of diagnosing a PTC in specimens with and without CLT. Source:
Prepared by the authors using Review Manager ® 5.3 software.
Table 1. Tiroiditis Linfocítica Crónica: Summary of the descriptive and the analytical
statistical analysis for each of the studies.
Discussion – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
The meta-analyses available in the literature, in which the authors attempt to gather evidence to suggest a causal association between CLT-compatible changes and the presence of PTC in surgical specimens resulting from thyroidectomies, have a common denominator: they suggest that the diagnosis of PTC is documented more frequently in specimens with CLT-compatible changes.
It seems to be a trend that after the pooled estimation of this risk, there is significant heterogeneity among the studies, even demonstrating the absence of a bias attributable to small studies; therefore, this heterogeneity should be interpreted as a wake-up call for those of us who dedicate ourselves to study this association.
The authors suggest that biases such as the high variability in the methodology of each of the studies, the absence of clear criteria for including the objects of study in the comparison groups and the absence of homogeneous criteria for indicating the surgical management of HD patients may be responsible for the heterogeneity.
However, in the present meta- analysis, in which a high homogeneity is clear as far as the methodological characteristics of the included studies are concerned, we obtained results like those published in the literature. Therefore, it is possible to conclude that there are sources of bias that have not been considered in the last 40 years, and furthermore, retrospective studies, and their limitations, will not allow us to go beyond suggesting a possible association.
Among the methodological limitations it is important to mention that, despite the retrospective nature of the studies:
None of them include a sample calculation, that is, they do not stipulate the minimum number of surgical specimens with and without CLT-compatible changes necessary to obtain results with an acceptable statistical power during the inferential analyses, and even in some series, which were excluded during the systematic review process, the authors do not give the reasons why they do not include all the specimens available during the period of time covered by the study.
Therefore, we suggest that in prospective studies the sample size should be determined by groups, to compare the proportion of specimens with PTC between a group of specimens with CLT and a group of specimens without CLT. Since we cannot prove a causal association, the hypothesis of independence should be of a bilateral nature and the dependent variable, corresponding to the finding of PTC in surgical specimens, should be of a qualitative, nominal and dichotomous nature.
When analyzing the prevalence of CTP in all the articles included (Table 1), it is possible to observe that a Berkson bias may be present in some series. The factors responsible for this bias could be the following:
It is striking that the prevalence of CTP in the studies by Siriwera et al, Buyukasik et al, Roberti et al, and Konturek et al are much lower than average, while in the studies by Zhang et al, Ye et al, and Giagourta et al, in contrast, they are much higher than average.
This may mean that, possibly, the institutions of the first group do not have an important annual volume of thyroidectomies, and that, in contrast, the institutions of the second group are reference centers for the management of thyroid cancer, so that undoubtedly, in centers whose population characteristics are similar to these groups, the sample size should be even larger than usual and even be collected over a longer time interval, to avoid sources of bias.
However, despite the proper calculation of sample sizes, it is possible that there may be difficulties in obtaining a significant number of specimens with changes compatible with CLT during the study period.
The results obtained in Table 1 allow us to assert that, on average, there is one specimen with changes compatible with CLT for every six specimens without changes compatible with CLT; this allows us to conclude that it is possible that this situation is configured as a selection bias, since patients who will have CLT in their specimen will be taken to surgery less frequently; therefore, situations such as the fact that changes compatible with CLT are more frequent in the group of specimens with PTC, that most of the patients with CLT will not undergo surgery in the development of the natural history of the disease, and that preoperative clinical suspicion of PTC might be, in the majority of cases, the reason that indicates their surgical management, may be the cause of the selection bias.
Conclusions – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
The current literature suggests that there is a higher risk of documenting a PTC in surgical specimens in which changes compatible with CLT are observed; however, there are sources of bias that will not be possible to control in retrospective studies, so we recommend studying the hypothesis that suggests a higher probability of diagnosing a PTC in specimens with changes compatible with CLT by means of prospective methodologies.
For this, possible sources of bias should be controlled by performing a sample size calculation based on the prevalence of PTC in specimens with and without CLT for each of the clinical scenarios to ensure real statistical power.
Finally, that the preoperative suspicion of a malignant neoplasm is, among others, the main reason indicating the performance of thyroidectomy in patients with or without clinical impression of an autoimmune thyroid disease, this fact will continue to behave as a constraint difficult to control and may be considered as the possible main source of bias on these studies.
Compliance with ethical standards
Informed consent: The present study was evaluated and approved by the Methodological and Ethical Committee from Research Subdivision of the E.S.E. Hospital Universitario del Caribe and registered under Institutional Code 46 – 22102020. In accordance with this committee and the Helsinki Declaration the request for informed consent was not required for the study to be carried out.
Conflict of interest: none declared by the authors.
Funding: The authors declare that this research was funded by the Vice-Rector’s Office for Research of the University of Cartagena, it was developed within the framework of the Scalpellum Research Group Strengthening Plan. Act of Commitment No. 021 of 2021 and Resolution No. 00416 of 2021.
Authors’ contribution
Conception and design of the study: Carlos Osorio-Covo.
Acquisition of data: Carlos Osorio-Covo, Jorge Ballestas- Barrera, Juan Correa-Palacio, Valeria Zambrano-Pacheco, Angie Rosales-Becerra, William Camargo-Martínez, Diego Barrios-Castellar, David Ortega-Caballero, Francisco Herrera-Saenz.
Data analysis and interpretation: Carlos Osorio-Covo, Jorge Ballestas-Barrera.
Drafting the manuscript: Carlos Osorio-Covo, Jorge Ballestas-Barrera.
Critical review: Carlos Osorio-Covo.
References – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
1. Rosai J, Albores Saavedra J, Asioli S, Baloch ZW, Bogdanova T, Chen H, et al. Papillary Thyroid Carcinoma. In: WHO Classification of Tumours of Endocrine Organs 4th edition. (Lloyd RV, Osamura RY, Klöppel G, Rosai J. eds.). IARC: Lyon; 2017. pp. 81-92.
2. Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. CA Cancer J Clin. 2013;63:11-30. https://doi.org/10.3322/caac.21166
3. Soares P, Celestino R, Gaspar da Rocha A, Sobrinho- Simões M. Papillary thyroid microcarcinoma: ¿How to diagnose and manage this epidemic? Int J Surg Pathol. 2014;22:113-9. https://doi.org/10.1177/1066896913517394
4. Siegel RL, Miller KD, Fuch HE, Jemal A. Cancer statistics 2022. CA Cancer J Clin. 2022;72:7-33. https://doi.org/10.3322/caac.21708
5. Bongiovanni M, Giovanella L, Romanelli F, Trimboli P. Cytological diagnosis associated with noninvasive follicular thyroid neoplasms with papillary-like nuclear features according to the Bethesda system for reporting thyroid cytopathology: A systematic review and meta-analysis. Thyroid. 2019;29:222-8. https://doi.org/10.1089/thy.2018.0394
6. Caturegli P, De Remigis A, Rose N. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13:391-7. https://doi.org/10.1016/j.autrev.2014.01.007
7. Huber G, Staub JJ, Meier C, Mitrache C, Guglielmetti M, Huber P, et al. Prospective study of the espontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab. 2002;87:3221-6. https://doi.org/10.1210/jcem.87.7.8678
8. Zaletel K, Gaberscek S. Hashimoto´s thyroiditis: From genes to the disease. Curr Genomics. 2011;12:576-88. https://doi.org/10.2174/138920211798120763
9. Nosé V. Hashimoto Thyroiditis. In: Diagnostic Pathology: Endocrine. 2nd edition (Nosé V, Cipriani N, Fisch A, eds). Canadá: Elsevier Health Sciences; 2018; pp 53-60.
Other References – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
10. Lindsay S, Dailey ME, Friedlander J, Yee G, Soley MG. Chronic thyroiditis: a clinical and pathologic study of 354 patients. J Clin Endocrinol Metab. 1952;12:1578- 1600. https://doi.org/10.1210/jcem-12-12-1578
11. Dailey M, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70:291-7. https://doi.org/10.1001/archsurg.1955.01270080137023
12. Holm L, Blomgren H, Löwhagen T. Cancer risk in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601-4. https://doi.org/10.1056/NEJM198503073121001
13. Anil C, Goksel S, Gursoy A. Hashimoto’s thyroiditis is not associated with increased risk of thyroid cancer in patients with thyroid nodules: a single-center prospective study. Thyroid. 2010;20:601-6. https://doi.org/10.1089/thy.2009.0450
14. Le K, Hershman J. Hashimoto´s thyroiditis does not predispose patients to differentiated thyroid cancer. Clin Thyroidology. 2014;26:227-9. https://doi.org/10.1089/ct.2014;26.227-229
15. Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999;126:1070‐7. https://doi.org/10.1067/msy.2099.101431
16. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol. 2013;168:343‐9. https://doi.org/10.1530/EJE-12-0903
17. Lai X, Xia Y, Zhang B, Li J, Jiang Y. A meta-analysis of Hashimoto’s thyroiditis and papillary thyroid carcinoma risk. Oncotarget. 2017;8:62414‐24. https://doi.org/10.18632/oncotarget.18620
18. Resende de Paiva C, Grønhøj C, Feldt-Rasmussen U, von Buchwald C. Association between Hashimoto’s thyroiditis and thyroid cancer in 64,628 patients. Front Oncol. 2017;7:53. https://doi.org/10.3389/fonc.2017.00053
Recommended readings – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
19. Jankovic B, Le K, Hershman J. Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013;98:474‐82. https://doi.org/10.1210/jc.2012-2978
20. Holm L, Blomgren H, Löwhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601‐4. https://doi.org/10.1056/NEJM19850307312100
21. Gul K, Dirikoc A, Kiyak G, Ersoy P, Ugras N, Ersoy R, et al. The association between thyroid carcinoma and Hashimoto’s thyroiditis: the ultrasonographic and histopathologic characteristics of malignant nodules. Thyroid. 2010;20:873‐8. https://doi.org/10.1089/thy.2009.0118
22. Alcântara-Jones D, Alcântara-Nunes T, Rocha B, Oliveira R, Santa A, Alcântara F, et al. Is there any association between Hashimoto’s thyroiditis and thyroid cancer? A retrospective data analysis. Radiol Bras. 2015;48:148‐53. https://doi.org/10.1590/0100-3984.2014.0072
23. Matesa-Anić D, Matesa N, Dabelić N, Kusić Z. Coexistence of papillary carcinoma and Hashimoto’s thyroiditis. Acta Clin Croat. 2009;48:9‐12.
24. Gómez J, Gómez N, Sahun M, Soler J. Prevalence and significance of lymphocyte infiltration in papillary carcinoma of the thyroid gland. Ann Med Int. 1997;14:403-5.
25. Asanuma K, Sugenoya A, Kasuga Y, Itoh N, Kobayashi S, Amano J. The relationship between multiple intrathyroidal involvement in papillary thyroid carcinoma and chronic non-specific thyroiditis. Cancer Lett. 1998;122:177‐80. https://doi.org/10.1016/s0304-3835(97)00398-4
26. Loh K, Greenspan F, Dong F, Miller T, Yeo P. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:458‐63. https://doi.org/10.1210/jcem.84.2.5443
27. Kebebew E, Treseler P, Ituarte P, Clark O. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg. 2001;25:632‐7. https://doi.org/10.1007/s002680020165
Other Recommended readings – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
28. Tamimi D. The association between chronic lymphocytic thyroiditis and thyroid tumors. Int J Surg Pathol. 2002;10:141‐6. https://doi.org/10.1177/106689690201000207
29. Cipolla C, Sandonato L, Graceffa G, Fricano S, Torcivia A, Vieni S, et al. Hashimoto thyroiditis coexistent with papillary thyroid carcinoma. Am Surg. 2005;71:874‐8. https://doi.org/10.1177/000313480507101018
30. Del Rio P, Cataldo S, Sommaruga L, Kim Y. The association between papillary carcinoma and chronic lymphocytic thyroiditis: does it modify the prognosis of cancer? Minerva Endocrinologica. 2008;33:1-5.
31. Muzza M, Degl’Innocenti D, Colombo C, Perrino M, Elena R, Rossi S, et al. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin Endocrinol (Oxf). 2010;72:702‐8. https://doi.org/10.1111/j.1365-2265.2009.03699.x
32. Huang B, Hseuh C, Chao T, Lin K, Lin J. Well-differentiated thyroid carcinoma with concomitant Hashimoto’s thyroiditis present with less aggressive clinical stage and low recurrence. Endocr Pathol. 2011;22:144‐9. https://doi.org/10.1007/s12022-011-9164-9
33. Yoon Y, Kim H, Lee J, Kim J, Koo B. The clinicopathologic differences in papillary thyroid carcinoma with or without co-existing chronic lymphocytic thyroiditis. Eur Arch Otorhinolaryngoly. 2012;269:1013-7. https://doi.org/10.1007/s00405-011-1732-6
34. Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700. https://doi.org/10.1136/bmj.b2700
35. Moher D, Liberati A, Tetzlaff J, Altman D, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:332-6. https://doi.org/10.1136/bmj.b2535
36. Dailey M, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg 1955;70:291-7. https://doi.org/10.1001/archsurg.1955.01270080137023
Bibliography – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
37. Okayasu I, Fujiwara M, Hara Y, Tanka Y, Rose N. Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese, and white and African Americans. Cancer. 1995;76:2312-8. https://doi.org/10.1002/1097-0142(19951201)76:11<2312::aid-cncr2820761120>3.0.co;2-h
38. Bradly DP, Reddy V, Prinz RA, Gattuso P. Incidental papillary carcinoma in patients treated surgically for benign thyroid diseases. Surgery 2009;146:1099-104. https://doi.org/10.1016/j.surg.2009.09.025
39. Consorti F, Loponte M, Milazzo F, Potasso L, Antonasi A. Risk of malignancy from thyroid nodular disease as an element of clinical management of patients with Hashimoto’s thyroiditis. Eur Surg Res 2010;45:333-7. https://doi.org/10.1159/000320954
40. Ahn D, Heo S, Park J, Kim J, Sohn J, Park J, et al. Clinical relationship between Hashimoto’s thyroiditis and papillary thyroid cancer. Acta Oncol 2011;50:1228-34. https://doi.org/10.3109/0284186X.2011.602109
41. Kim K, Park Y, Kim E, Park S, Park D, Ahn S, et al. Elevated risk of papillary thyroid cancer in Korean patients with Hashimoto’s thyroiditis. Head Neck. 2011;33:691- 5. https://doi.org/10.1002/hed.21518
42. Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z, et al. Hashimoto’s thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngol Head Neck Surg. 2013;148:396-402. https://doi.org/10.1177/0194599812472426
43. Kutluturk K, Unal B, Cetin S. Coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma: a single center experience. J Turgut Ozal Med Cent. 2017;24:190-2. https://doi.org/10.5455/jtomc.2017.03.34
44. Lee I, Hsieh A, Lee T, Lee T, Chien Y. The association of thyrotropin and autoimmune thyroid disease in developing papillary thyroid cancer. Int J Endocrinol. 2017;2017:5940367. https://doi.org/10.1155/2017/5940367
45. Nawarathna N, Ratnayake P, Hewage S, Senevirathne J, Gunatilake S, Kariyawasam N, et al. Association between nonspecific chronic lymphocytic thyroiditis and differentiated epithelial thyroid carcinoma: Clinicopathological analysis of patients who underwent thyroidectomy in a tertiary care center in Sri Lanka. World J Endoc Surg. 2018;10:119-26. https://doi.org/10.5005/jp-journals-10002-1230
other Bibliography – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
46. Jackson D, Handelsman R, Farrá J, Lew J. Increased incidental thyroid cancer in patients with subclinical chronic lymphocytic thyroiditis. J Surg Res. 2020;245:115-8. https://doi.org/10.1016/j.jss.2019.07.025
47. Peterson C, Shidler F. Lymphocytic thyroiditis in 757 thyroid operations. Am J Surg. 1957;94:223-31. https://doi.org/10.1016/0002-9610(57)90649-9
48. Shands W. Carcinoma of the thyroid in association with struma lymphomatosa (Hashimoto’s disease). Ann Surg. 1960;151:675-82. https://doi.org/10.1097/00000658-196005000-00008
49. Campos L, Picado S, Guimarães A, Ribeiro D, Dedivitis R. Thyroid papillary carcinoma associated to Hashimoto’s thyroiditis. Braz J Otorhinolaryngol. 2012;78:77-80. https://doi.org/10.5935/1808-8694.20120037
50. Yamashita H, Nakayama I, Noguchi S, Murakami N, Moriuchi A, Yokoyama S, et al. Thyroid carcinoma in benign thyroid diseases. An analysis from minute carcinoma. Acta Pathol Jpn. 1985;35:781-8. https://doi.org/10.1111/j.1440-1827.1985.tb00620.x
51. McLeod M, East M, Burney R, Harness J, Thompson N. Hashimoto’s thyroiditis revisited: the association with thyroid cancer remains obscure. World J Surg. 1988;12:509-6. https://doi.org/10.1007/BF01655435
52. Roberti A, Sobrinho J, Porto O, Rapoport A. Concomitância da tireoidite de Hashimoto e o carcinoma diferenciado da tireóide. Rev Col Bras Cir. 2006;33:345-9. https://doi.org/10.1590/S0100-69912006000600003
53. Kurukahvecioglu O, Taneri F, Yüksel O, Aydin A, Tezel E, Onuk E. Total thyroidectomy for the treatment of Hashimoto’s thyroiditis coexisting with papillary thyroid carcinoma. Adv Ther. 2007;24:510-6. https://doi.org/10.1007/BF02848773
54. Larson S, Jackson L, Riall T, Uchida T, Thomas R, Qiu S, et al. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg. 2007;204:764-75. https://doi.org/10.1016/j.jamcollsurg.2006.12.037
Bibliographical sources – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
55. Repplinger D, Bargren A, Zhang YW, Adler JT, Haymart M, Chen H. Is Hashimoto’s thyroiditis a risk factor for papillary thyroid cancer? J Surg Res. 2008;150:49-52. https://doi.org/10.1016/j.jss.2007.09.020
56. Mazokopakis E, Tzortzinis A, Dalieraki-Ott E, Tsartsalis A, Syros P, Karefilakis C, et al. Coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma. A retrospective study. Hormones (Athens). 2010;9:312-7. https://doi.org/10.14310/horm.2002.1282
57. Siriweera E, Ratnatunga N. Profile of Hashimoto’s thyroiditis in Sri Lankans: Is there an increased risk of ancillary pathologies in Hashimoto’s thyroiditis? J Thyroid Res. 2010;2010:124264. https://doi.org/10.4061/2010/124264
58. Buyukasik O, Hasdemir A, Yalcin E, Celep B, Sengul S, Yandakci K, et al. The association between thyroid malignancy and chronic lymphocytic thyroiditis: should it alter the surgical approach? Endokrynol Pol. 2011; 4:303-8.
59. Zhang L, Li H, Ji Q, Zhu Y,Wang Z, Wang Y,et al. The clinical features of papillary thyroid cancer in Hashimoto’s thyroiditis patients from an area with a high prevalence of Hashimoto’s disease. BMC Cancer. 2012;12:610. https://doi.org/10.1186/1471-2407-12-610
60. Konturek A, Barczyński M, Wierzchowski W, Stopa M, Nowak W. Coexistence of papillary thyroid cancer with Hashimoto thyroiditis. Langenbecks Arch Surg. 2013;398:389-94. https://doi.org/10.1007/s00423-012-1021-x
61. Ye Z, Gu D, Hu H, Zhou Y, Hu X, Zhang X. Hashimoto’s thyroiditis, microcalcification and raised thyrotropin levels within normal range are associated with thyroid cancer. World J Surg Oncol. 2013;11:56. https://doi.org/10.1186/1477-7819-11-56
62. Giagourta I, Evangelopoulou C, Papaioannou G, Kassi G, Zapanti E, Prokopiou M, et al. Autoimmune thyroiditis in benign and malignant thyroid nodules: 16-year results. Head Neck. 2014;36:531-5. https://doi.org/10.1002/hed.23331 49 Rev Colomb Cir. 2023;38:37-49 Association between lymphocityc thyroiditis and thyroid carcinoma
63. Liu X, Zhu L, Cui D, Wang Z, Chen H, Duan Y, et al. Coexistence of histologically confirmed Hashimoto’s thyroiditis with different stages of papillary thyroid carcinoma in a consecutive chinese cohort. Int J Endocrinol. 2014;2014:769294. https://doi.org/10.1155/2014/769294
Other Bibliographical sources – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
64. Paparodis R, Imam S, Todorova-Koteva K, Staii A, Jaume J. Hashimoto’s thyroiditis pathology and risk for thyroid cancer. Thyroid. 2014;24:1107-14. https://doi.org/10.1089/thy.2013.0588
65. Zayed A, Ali M, Jaber O, Suleiman M, Ashhab A, Al Shweiat W, et al. Is Hashimoto’s thyroiditis a risk factor for medullary thyroid carcinoma? Our experience and a literature review. Endocrine. 2015;48:629-36. https://doi.org/10.1007/s12020-014-0363-2
66. Zhang Y, Ma X, Deng F, Liu Z, Wei H, Wang X, et al. The effect of chronic lymphocytic thyroiditis on patients with thyroid cancer. World J Surg Oncol. 2014;12:277. https://doi.org/10.1186/1477-7819-12-277
67. Zhang Y, Dai J, Wu T, Yang N, Yin Z. The study of the coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2014;140:1021-6. https://doi.org/10.1007/s00432-014-1629-z
68. Gabalec F, Srbova L, Nova M, Hovorkova E, Hornychova H, Jakubikova I, et al. Impact of Hashimoto’s thyroiditis, TSH levels, and anti-thyroid antibody positivity on differentiated thyroid carcinoma incidence. Endokrynol Pol. 2016;67:48-53. https://doi.org/10.5603/EP.a2016.0022
69. Liang J, Zeng W, Fang F, Yu T, Zhao Y, Fan X, et al. Clinical analysis of Hashimoto thyroiditis coexistent with papillary thyroid cancer in 1392 patients. Acta Otorhinolaryngol Ital. 2017;37:393-400. https://doi.org/10.14639/0392-100X-1709
70. Uhliarova B, Hajtman A. Hashimoto’s thyroiditis – an independent risk factor for papillary carcinoma. Braz J Otorhinolaryngol. 2018;84:729-35. https://doi.org/10.1016/j.bjorl.2017.08.012
71. Osorio C, Ibarra S, Arrieta J, Sarmiento M, Barrios D, Sierra L, et al. Association between chronic lymphocytic thyroiditis and papillary thyroid carcinoma: A retrospective study in surgical specimens. Rev Esp Patol. 2020;53:149-57. https://doi.org/10.1016/j.patol.2019.07.004
Authors – Asociación entre Tiroiditis Linfocítica Crónica y Carcinoma Papilar de Tiroides
1 Carlos Osorio-Covo, Francisco Herrera-Sáenz, MD, General Surgeon, Department of General Surgery, Unit of Endocrine Surgery, Caribbean University Hospital. Professor, Scalpellum Research Group, Faculty of Medicine, University of Cartagena, Cartagena, Colombia.
2 Jorge Ballestas-Barrera , Juan Correa-Palacio, MD, General Surgery Undergraduate Student. Scalpellum Research Group, Faculty of Medicine, University of Cartagena. Cartagena, Colombia.
3 Valeria Zambrano-Pacheco, Angie Rosales-Becerra, William Camargo-Martínez, Medical Student, University of Cartagena, Scalpellum Research Seedbed, Cartagena, Colombia.
4 Diego Barrios-Castellar, David Ortega-Caballero, MD, Scalpellum Research Group, Faculty of Medicine, University of Cartagena. Cartagena, Colombia.
Received: 3/08/2022 – Accepted: 25/09/2022 – Published online: 23/12/2022
Corresponding author: Carlos Osorio-Covo, E.S.E. Hospital Universitario del Caribe, 29th Street # 50-50, Zaragocilla, Cartagena, Colombia. Tel.: + 57 3194988374. Email: cosorioc@gruposcalpellum.com
Cite as: Osorio-Covo C, Ballestas-Barrera J, Correa-Palacio J, Zambrano-Pacheco V, Rosales-Becerra A, Camargo-Martinez W, et al. Asociación entre tiroiditis linfocítica crónica y carcinoma papilar de tiroides: Revisión sistemática y metaanálisis de estudios en especímenes quirúrgicos. Rev Colomb Cir. 2023;38:37-49. https://doi.org/10.30944/20117582.2228
This is an open Access under a Creative Commons License – BY-NC-ND https://creativecommons.org/licenses/by-ncnd/4.0/deed.es