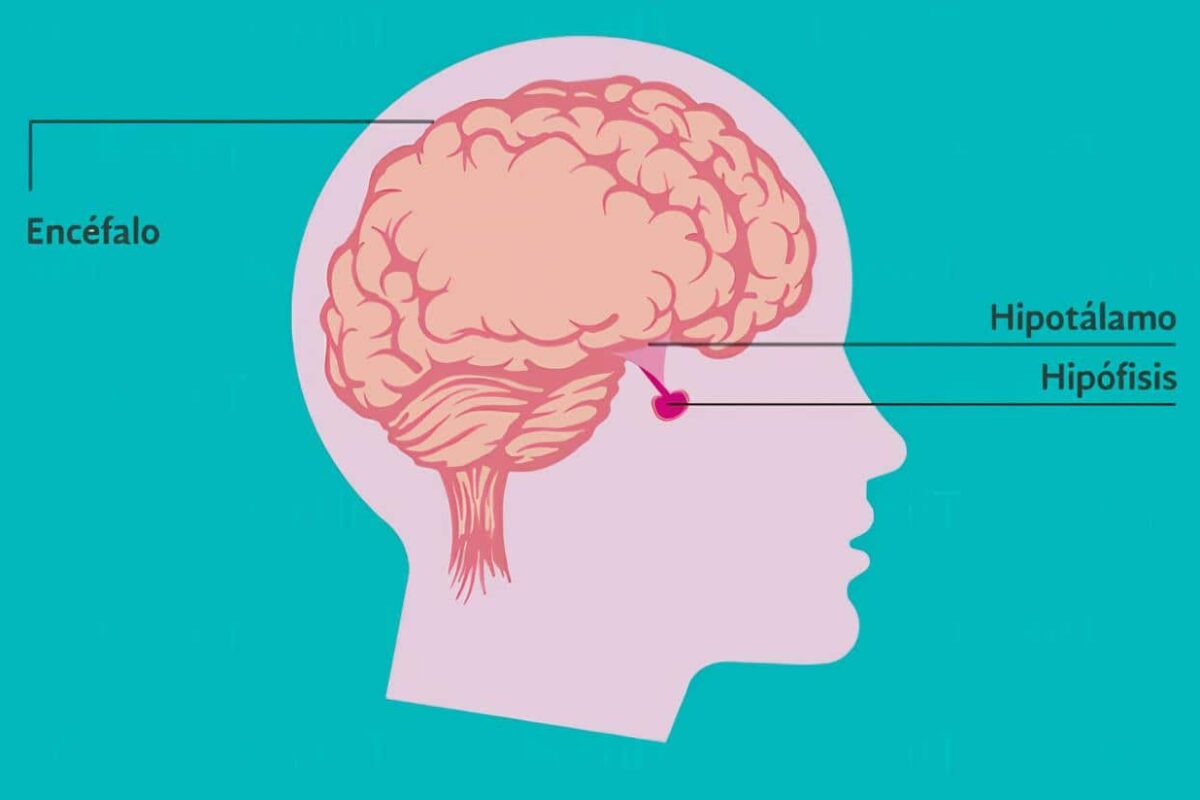
¿Cómo encaja la evidencia con el entendimiento biológico?
El tamoxifeno y el raloxifeno
El tamoxifeno y el raloxifeno son moduladores de RE selectivos que inhiben las RE en el tejido mamario y reducen el riesgo de cáncer de mama positivo a RE al bloquear la proliferación de células epiteliales sensibles al estrógeno donde se puede desarrollar cáncer de mama. Dichos medicamentos han sido aprobados por la Administración de Drogas y Alimentos de los Estados Unidos. Para la reducción del riesgo de cáncer de mama.
Los inhibidores de la aromatasa inhiben la conversión de andrógeno a estrógeno y pueden reducir el riesgo de cáncer de mama ER-positivo al disminuir la cantidad de estrógeno disponible para unirse a las células epiteliales sensibles al estrógeno.
Los inhibidores de la aromatasa han sido evaluados para la reducción del riesgo de cáncer de mama en ensayos clínicos. Aunque se utilizan principalmente para el tratamiento en lugar de la reducción del riesgo de cáncer primario. De igual manera, los inhibidores de la aromatasa no están aprobados actualmente por la Administración de Medicamentos y Alimentos de los Estados Unidos. Para la reducción del riesgo de cáncer de mama primario.92-93
Referencias
-
1. National Cancer Institute. SEER cancer stat facts: female breast cancer. https://seer.cancer.gov/statfacts/html/breast.html This link goes offsite. Click to read the external link disclaimer. Accessed December 19, 2018.
-
2. American Cancer Society. Cancer facts & figures 2018. https://www.cancer.org/research/cancer-facts-statistics/all-cancerfacts-figures/cancer-facts-figures-2018.html This link goes offsite. Click to read the external link disclaimer. Accessed December 19, 2018.
-
3. National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/This link goes offsite. Click to read the external link disclaimer. Accessed December 19, 2018.
-
4. Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29(17):2327-33.
-
5. Nelson HD, Fu R, Zakher B, et al. Medication Use for the Risk Reduction of Primary Breast Cancer in Women: A Systematic Review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 180. AHRQ Publication No. 19-05249-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2019.
-
6. Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97(19):1428-37.
-
7. Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402-16.
-
8. American Cancer Society. Breast Cancer Facts & Figures: 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancerfacts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf This link goes offsite. Click to read the external link disclaimer. Accessed December 19, 2018.
-
9. National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer/causesprevention/genetics/brca-fact-sheet This link goes offsite. Click to read the external link disclaimer. Accessed December 19, 2018.
-
10. Forbes JF, Sestak I, Howell A, et al; IBIS-II Investigators. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomized controlled trial. Lancet. 2016; 387(10021):866-73.
-
11. Goldvaser H, Barnes TA, Šeruga B, et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(1).
-
12. Cuzick J, Forbes JF, Sestak I, et al; International Breast Cancer Intervention Study I Investigators. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99(4):272-82.
-
13. Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99(4):283-90.
-
14. Cuzick J, Sestak I, Cawthorn S, et al; IBIS-I Investigators. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16(1):67-75.
-
15. U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279-96.
-
16. U.S. Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271-81.
-
17. National Cancer Institute. Breast cancer prevention (PDQ®)–patient version. https://www.cancer.gov/types/breast/patient/breast-prevention-pdq This link goes offsite. Click to read the external link disclaimer. Accessed December 19, 2018.
-
18. Centers for Disease Control and Prevention. What can I do to reduce my risk of breast cancer? https://www.cdc.gov/cancer/breast/basic_info/prevention.htm This link goes offsite. Click to read the external link disclaimer. Accessed December 19, 2018.
-
19. Breast Cancer Surveillance Consortium. Breast Cancer Surveillance Consortium Risk Calculator. https://tools.bcsc-scc.org/bc5yearrisk/calculator.htm This link goes offsite. Click to read the external link disclaimer. Accessed December 19, 2018.
-
20. Kaplan CP, Haas JS, Pérez-Stable EJ, Des Jarlais G, Gregorich SE. Factors affecting breast cancer risk reduction practices among California physicians. Prev Med. 2005;41(1):7-15.
-
21. Corbelli J, Borrero S, Bonnema R, et al. Use of the Gail model and breast cancer preventive therapy among three primary care specialties. J Womens Health (Larchmt). 2014;23(9):746-52.
-
22. Armstrong K, Quistberg DA, Micco E, Domchek S, Guerra C. Prescription of tamoxifen for breast cancer prevention by primary care physicians. Arch Intern Med. 2006;166(20):2260-5.
-
23. Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575-90.
-
24. Fagerlin A, Zikmund-Fisher BJ, Nair V, et al. Women’s decisions regarding tamoxifen for breast cancer prevention: responses to a tailored decision aid. Breast Cancer Res Treat. 2010;119(3):613-20.
-
25. Melnikow J, Paterniti D, Azari R, et al. Preferences of Women Evaluating Risks of Tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer. 2005;103(10):1996-2005.
-
26. McKay A, Martin W, Latosinsky S. How should we inform women at higher risk of breast cancer about tamoxifen? An approach with a decision guide. Breast Cancer Res Treat. 2005;94(2):153-9.
-
27. Paterniti DA, Melnikow J, Nuovo J, et al. «I’m going to die of something anyway»: women’s perceptions of tamoxifen for breast cancer risk reduction. Ethn Dis. 2005;15(3):365-72.
-
28. Fagerlin A, Dillard AJ, Smith DM, et al. Women’s interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast Cancer Res Treat. 2011;127(3):681-8.
-
29. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879-86.
-
30. Adams-Campbell LL, Makambi KH, Palmer JR, Rosenberg L. Diagnostic accuracy of the Gail model in the Black Women’s Health Study. Breast J. 2007;13(4):332-6.
-
31. Chen J, Pee D, Ayyagari R, et al. Projecting absolute invasive breast cancer risk in white women with a model that includes mammographic density. J Natl Cancer Inst. 2006;98(17): 1215-26.
-
32. Costantino JP, Gail MH, Pee D, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91(18):1541-8.
-
33. Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99(23):1782-92.
-
34. Matsuno RK, Costantino JP, Ziegler RG, et al. Projecting individualized absolute invasive breast cancer risk in Asian and Pacific Islander American women. J Natl Cancer Inst. 2011;103(12):951-61.
-
35. Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001;93(5):358-66.
-
36. Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008; 148(5):337-47.
-
37. Barlow WE, White E, Ballard-Barbash R, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006; 98(17):1204-14.
-
38. Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33(28):3137-43.
-
39. Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses’ Health Study. Am J Epidemiol. 2000;152(10):950-64.
-
40. Rockhill B, Byrne C, Rosner B, Louie MM, Colditz G. Breast cancer risk prediction with a log-incidence model: evaluation of accuracy. J Clin Epidemiol. 2003;56(9):856-61.
-
41. Tamimi RM, Rosner B, Colditz GA. Evaluation of a breast cancer risk prediction model expanded to include category of prior benign breast disease lesion. Cancer. 2010; 116(21):4944-53.
-
42. Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004; 96(3):218-28.
-
43. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004; 23(7):1111-30.
-
44. Amir E, Evans DG, Shenton A, et al. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J Med Genet. 2003; 40(11):807-14.
-
45. Boughey JC, Hartmann LC, Anderson SS, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28(22):3591-6.
-
46. Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res. 2015;17(1):147.
-
47. Chlebowski RT, Anderson GL, Lane DS, et al; Women’s Health Initiative Investigators. Predicting risk of breast cancer in postmenopausal women by hormone receptor status. J Natl Cancer Inst. 2007;99(22):1695-705.
-
48. Boyle P, Mezzetti M, La Vecchia C, Franceschi S, Decarli A, Robertson C. Contribution of three components to individual cancer risk predicting breast cancer risk in Italy. Eur J Cancer Prev. 2004;13(3):183-91.
-
49. Decarli A, Calza S, Masala G, Specchia C, Palli D, Gail MH. Gail model for prediction of absolute risk of invasive breast cancer: independent evaluation in the Florence-European Prospective Investigation Into Cancer and Nutrition Cohort. J Natl Cancer Inst. 2006;98(23):1686-93.
-
50. Petracci E, Decarli A, Schairer C, et al. Risk factor modification and projections of absolute breast cancer risk. J Natl Cancer Inst. 2011;103(13):1037-48.
-
51. Cuzick J, Forbes J, Edwards R, et al; IBIS Investigators. First results from the International Breast Cancer Intervention Study (IBISI): a randomised prevention trial. Lancet. 2002;360(9336):817-24.
-
52. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371-88.
-
53. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652-62.
-
54. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) randomized study. J Natl Cancer Inst. 2001;93(21):1615-23.
-
55. Powles T, Eeles R, Ashley S, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial. Lancet. 1998;352(9122):98-101.
-
56. Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet. 1998;352(9122):93-7.
-
57. Veronesi U, Maisonneuve P, Rotmensz N, et al; Italian Tamoxifen Study Group. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007; 99(9):727-37.
-
58. Veronesi U, Maisonneuve P, Rotmensz N, et al; Italian Tamoxifen Study Group. Italian randomized trial among women with hysterectomy: tamoxifen and hormonedependent breast cancer in high-risk women. J Natl Cancer Inst. 2003;95(2):160-5.
-
59. Decensi A, Maisonneuve P, Rotmensz N, et al; Italian Tamoxifen Study Group. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation. 2005;111(5):650-6.
-
60. DeCensi A, Bonanni B, Maisonneuve P, et al; Italian HOT Study Group. A phase-III prevention trial of low-dose tamoxifen in postmenopausal hormone replacement therapy users: the HOT study. Ann Oncol. 2013;24(11):2753-60.
-
61. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637-45.
-
62. Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281(23):2189-97.
-
63. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat. 2001; 65(2):125-34.
-
64. Barrett-Connor E, Grady D, Sashegyi A, et al; MORE Investigators (Multiple Outcomes of Raloxifene Evaluation). Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002;287(7):847-57.
-
65. Delmas PD, Ensrud KE, Adachi JD, et al; Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87(8):3609-17.
-
66. Delmas PD, Genant HK, Crans GG, et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone. 2003;33(4):522-32.
-
67. Grady D, Ettinger B, Moscarelli E, et al; Multiple Outcomes of Raloxifene Evaluation Investigators. Safety and adverse effects associated with raloxifene: multiple outcomes of raloxifene evaluation. Obstet Gynecol. 2004;104(4):837-44.
-
68. Barrett-Connor E, Cauley JA, Kulkarni PM, Sashegyi A, Cox DA, Geiger MJ. Risk-benefit profile for raloxifene: 4-year data from the Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial. J Bone Miner Res. 2004;19(8):1270-5.
-
69. Silverman SL, Delmas PD, Kulkarni PM, Stock JL, Wong M, Plouffe L Jr. Comparison of fracture, cardiovascular event, and breast cancer rates at 3 years in postmenopausal women with osteoporosis. J Am Geriatr Soc. 2004;52(9):1543-8.
-
70. Martino S, Disch D, Dowsett SA, Keech CA, Mershon JL. Safety assessment of raloxifene over eight years in a clinical trial setting. Curr Med Res Opin. 2005;21(9):1441-52.
-
71. Duvernoy CS, Kulkarni PM, Dowsett SA, Keech CA. Vascular events in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial: incidence, patient characteristics, and effect of raloxifene. Menopause. 2005;12(4):444-52.
-
72. Keech CA, Sashegyi A, Barrett-Connor E. Year-by-year analysis of cardiovascular events in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial. Curr Med Res Opin. 2005;21(1):135-140.
-
73. Siris ES, Harris ST, Eastell R, et al; Continuing Outcomes Relevant to Evista (CORE) Investigators. Skeletal effects of raloxifene after 8 years: results from the continuing outcomes relevant to Evista (CORE) study. J Bone Miner Res. 2005;20(9):1514-24.
-
74. Lippman ME, Cummings SR, Disch DP, et al. Effect of raloxifene on the incidence of invasive breast cancer in postmenopausal women with osteoporosis categorized by breast cancer risk. Clin Cancer Res. 2006; 12(17):5242-7.
-
75. Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355(2):125-37.
-
76. Ensrud KE, Stock JL, Barrett-Connor E, et al. Effects of raloxifene on fracture risk in postmenopausal women: the Raloxifene Use for the Heart Trial. J Bone Miner Res. 2008;23(1):112-20.
-
77. Grady D, Cauley JA, Geiger MJ, et al; Raloxifene Use for The Heart Trial Investigators. Reduced incidence of invasive breast cancer with raloxifene among women at increased coronary risk. J Natl Cancer Inst. 2008;100(12):854-61.
-
78. Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2742-51.
-
79. Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P- 2 trial. JAMA. 2006;295(23):2727-41.
-
80. Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3(6):696-706.
-
81. Maunsell E, Goss PE, Chlebowski RT, et al. Quality of life in MAP.3 (Mammary Prevention 3): a randomized, placebo-controlled trial evaluating exemestane for prevention of breast cancer. J Clin Oncol. 2014;32(14):1427-36.
-
82. Goss PE, Ingle JN, Alés-Martínez JE, et al; NCIC CTG MAP.3 Study Investigators. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381-91.
-
83. Cuzick J, Sestak I, Forbes JF, et al; IBIS-II Investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, doubleblind, randomised placebo-controlled trial. [Erratum appears in Lancet. 2014;383(9922): 1040]. Lancet. 2014;383(9922):1041-8.
-
84. Sestak I, Singh S, Cuzick J, et al. Changes in bone mineral density at 3 years in postmenopausal women receiving anastrozole and risedronate in the IBIS-II bone substudy: an international, double-blind, randomised, placebo-controlled trial. [Erratum appears in Lancet Oncol. 2014;15(13): e587]. Lancet Oncol. 2014;15(13):1460-8.
-
85. Spagnolo F, Sestak I, Howell A, Forbes JF, Cuzick J. Anastrozole-induced carpal tunnel syndrome: results from the International Breast Cancer Intervention Study II Prevention Trial. J Clin Oncol. 2016;34(2):139-43.
-
86. Golvaser H, Barnes TA, Šeruga B, et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110(1).
-
87. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patientlevel meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341-52
-
88. U.S. Preventive Services Task Force. Medications to decrease the risk for breast cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708.
-
89. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2013;31(23):2942-62.
-
90. Bevers TB, Ward JH, Arun BK, et al. Breast Cancer Risk Reduction, Version 2.2015. J Natl Compr Canc Netw. 2015;13(7):880-915.
-
91. Committee on Practice Bulletins–Gynecology, Committee on Genetics, Society of Gynecologic Oncology. Practice Bulletin No. 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110-e126.
-
92. Committee Opinion No. 601: tamoxifen and uterine cancer. Obstet Gynecol. 2014;123(6): 1394-7.
-
93. American Academy of Family Physicians. Clinical preventive service recommendation: medications to reduce breast cancer risk. https://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancermedication.html This link goes offsite. Click to read the external link disclaimer. Accessed December 19, 2018.