Biopsia Líquida
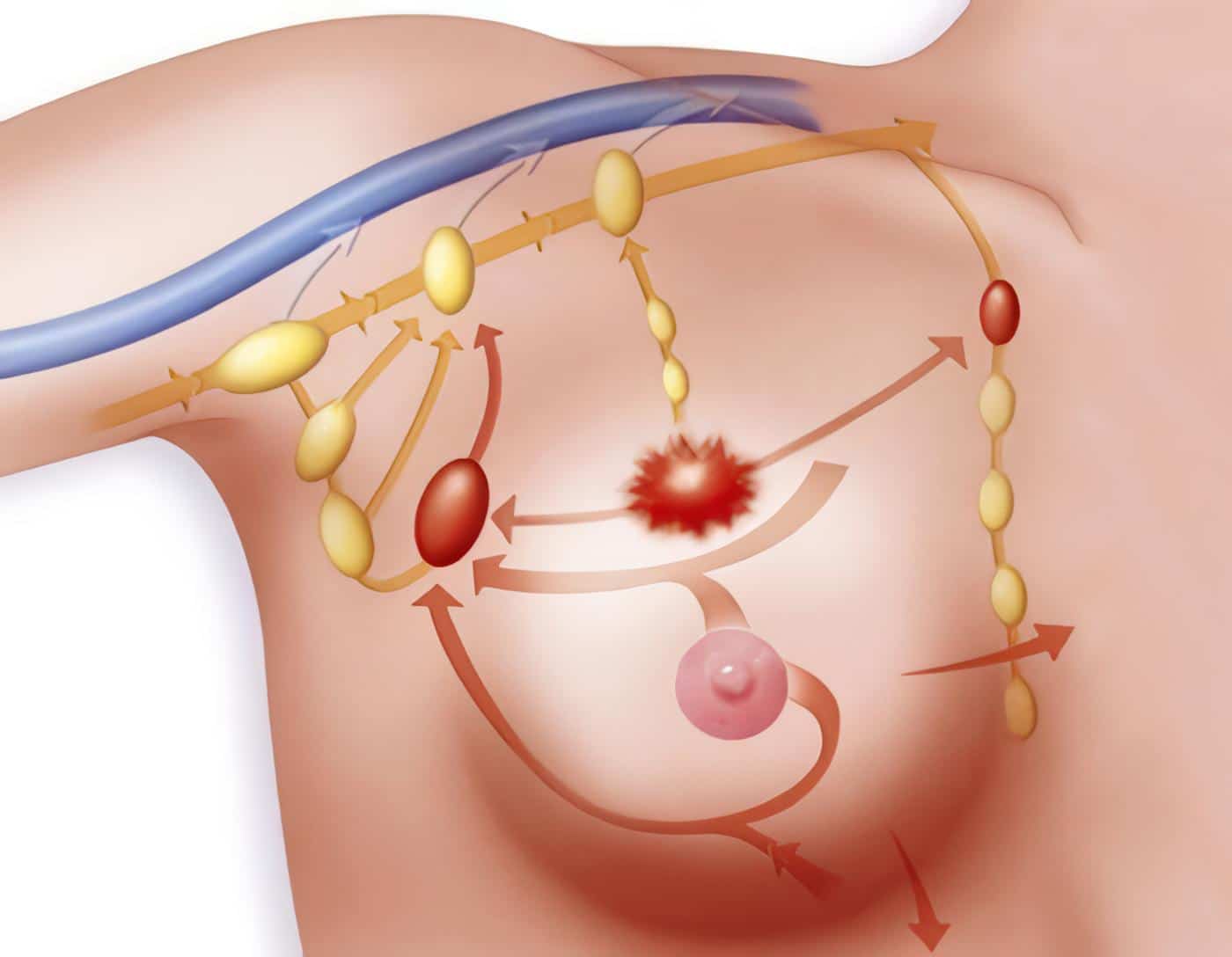
La identificación de células tumorales circulantes se convirtió en un objetivo importante del estudio de la biología de las metástasis, luego ocurriría lo mismo con fragmentos circulantes de las células neoplásicas denominados exosomas, resultantes de procesos de apoptosis, para los cuales se postuló un papel importante en el preacondicionamiento de los nichos metastásicos.
Luego hubo un enfoque específico sobre fracciones de ácidos nucleicos libres como DNA y diferentes tipos de RNAs de células tumorales en la circulación (Figura 5); en su sentido más amplio, el concepto de biopsia líquida, término acuñado en 2010 (138), aunque, el concepto empezara a usarse algunos años antes (139), abarca el estudio además de la sangre de otros líquidos corporales como pleural, peritoneal, cefalorraquídeo, orina etc.
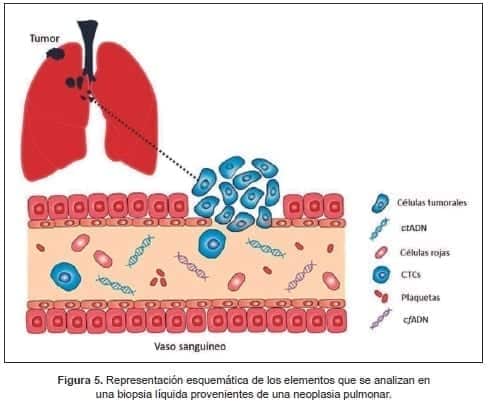
El estudio de estos elementos aporta información adicional sobre la heterogeneidad tumoral, por presentar frecuentemente características moleculares diferentes de los tumores primarios e incluso de las metástasis (138- 140).
El limitado número de estos elementos circulantes planteó un importante reto para su estudio, que llevó inicialmente al desarrollo de metodologías para su aislamiento diferencial de elementos circulantes similares provenientes de elementos celulares no neoplásicos, que se basa en el uso de métodos inmunocitoquímicos para aislar los mediante anticuerpos dirigidos contra antígenos de células epiteliales (141, 142).
La evaluación de pacientes con recidivas recurrentes de neoplasias
La evaluación de pacientes con recidivas recurrentes de neoplasias que habitualmente requieren de la obtención de varias biopsias para su demostración y la necesidad de estudiar factores asociados a resistencia al tratamiento, encontraría en el estudio de estos elementos circulantes una alternativa, que dio lugar al desarrollo de la biopsia líquida, técnica que se basa en su evaluación mediante pruebas de PCR y secuenciación profunda (143-147).
Esta metodología además de sus ventajas por no ser invasiva, permite efectuar seguimiento a largo plazo de los pacientes, evaluando información genómica, transcriptómica y epigenetica relacionada con la heterogeneidad tumoral, para brindar a las pacientes alternativas terapéuticas cuando se detecten nuevas alteraciones moleculares que provean a los tumores de ventajas proliferativas, de escape de la respuesta inmune o asociadas a resistencia los tratamientos (141, 142, 148).
En la actualidad la biopsia líquida ya se utiliza rutinariamente en el estudio de pacientes con tumores metastásicos de seno (149, 150), pulmón (151, 152), colon (153, 154), páncreas (155), melanomas (156) entre otros y sus indicaciones ya han empezado a ser reguladas (157).
En el futuro, incluso se plantea la posibilidad de que la biopsia líquida haga parte del abordaje diagnóstico inicial de pacientes con cáncer junto con las biopsias de tejidos, e inclusive reemplazándolas en algunas situaciones como tumores de difícil acceso (142, 158, 159).
Referencias
-
1. Coons AH, Creech HJ, Jones RN. Immunological Properties of an Antibody Containing a Fluorescent Group. Exper Biol Med. 1941;47(2):200-2.
-
2. Taylor CR. Immunoenzyme techniques and their application to diagnostic studies. Ann N Y Acad Sci. 1983;420:115-26.
-
3. Huang SN, Minassian H, More JD. Application of immunofluorescent staining on paraffin sections improved by trypsin digestion. Lab Invest. 1976;35(4):383-90.
-
4. Nakane PK, Pierce GB, Jr. Enzyme-labeled antibodies for the light and electron microscopic localization of tissue antigens. J Cell Biol. 1967;33(2):307-18.
-
5. Mason TE, Phifer RF, Spicer SS, Swallow RA, Dreskin RB. An immunoglobulin-enzyme bridge method for localizing tissue antigens. J Histochem Cytochem. 1969;17(9):563-9.
-
6. Avrameas S. Enzyme markers: their linkage with proteins and use in immuno-histochemistry. Histochem J. 1972;4(4):321-30.
-
7. Taylor CR, Burns J. The demonstration of plasma cells and other immunoglobulin-containing cells in formalinfixed, paraffin-embedded tissues using peroxidase-labelled antibody. J Clin Pathol. 1974;27(1):14-20.
-
8. Pinkus GS. Diagnostic immunocytochemistry of paraffinembedded tissues. Hum Pathol. 1982;13(5):411-5. 9. Burns J. Background staining and sensitivity of the unlabelled antibody-enzyme (PAP) method. Comparison with the peroxidase labelled antibody sandwich method using formalin fixed paraffin embedded material. Histochemistry. 1975;43(3):291-4.
-
10. Taylor CR. An exaltation of experts: concerted efforts in the standardization of immunohistochemistry. Hum Pathol. 1994;25(1):2-11.
-
11. Burry RW. Specificity controls for immunocytochemical methods. J Histochem Cytochem. 2000;48(2):163-6.
-
12. Torlakovic EE, Nielsen S, Francis G, Garratt J, Gilks B, Goldsmith JD et al. Standardization of positive controls in diagnostic immunohistochemistry: recommendations from the International Ad Hoc Expert Committee. Appl Immunohistochem Mol Morphol. 2015;23(1):1-18.
-
13. Torlakovic EE, Francis G, Garratt J, Gilks B, Hyjek E, Ibrahim M et al. Standardization of negative controls in diagnostic immunohistochemistry: recommendations from the international ad hoc expert panel. Appl Immunohistochem Mol Morphol. 2014;22(4):241-52.
-
14. Mason JT, O’Leary TJ. Effects of formaldehyde fixation on protein secondary structure: a calorimetric and infrared spectroscopic investigation. J Histochem Cytochem. 1991;39(2):225-9.
-
15. Werner M, Chott A, Fabiano A, Battifora H. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol. 2000;24(7):1016-9.
-
16. Leong AS, Gilham PN. The effects of progressive formaldehyde fixation on the preservation of tissue antigens. Pathology. 1989;21(4):266-8.
-
17. Vincek V, Nassiri M, Nadji M, Morales AR. A Tissue Fixative that Protects Macromolecules (DNA, RNA, and Protein) and Histomorphology in Clinical Samples. Lab Invest. 2003;83(10):1427-35.
-
18. Wester K, Asplund A, Bäckvall H, Micke P, Derveniece A, Hartmane I et al. Zinc-based fixative improves preservation of genomic DNA and proteins in histoprocessing of human tissues. Lab Invest. 2003;83(6):889-99.
-
19. Shibutani M, Uneyama C, Miyazaki K, Toyoda K, Hirose M. Methacarn Fixation: A Novel Tool for Analysis of Gene Expressions in Paraffin-Embedded Tissue Specimens. Lab Invest. 2000;80(2):199-208.
-
20. Prentø P, Lyon H. Commercial formalin substitutes for histopathology. Biotech Histochem. 1997;72(5):273-82.
-
21. Sternberger LA, Hardy PH, Jr., Cuculis JJ, Meyer HG. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidaseantihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970;18(5):315- 33.
-
22. DeLellis RA, Sternberger LA, Mann RB, Banks PM, Nakane PK. Immunoperoxidase technics in diagnostic pathology. Report of a workshop sponsored by the National Cancer Institute. Am J Clin Pathol. 1979;71(5):483-8.
-
23. Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29(4):577-80.
-
24. Levsky JM, Singer RH. Fluorescence in situ hybridization: past, present and future. J Cell Sci. 2003;116(Pt 14):2833-8.
-
25. Streefkerk JG. Inhibition of erythrocyte pseudoperoxidase activity by treatment with hydrogen peroxide following methanol. J Histochem Cytochem. 1972;20(10):829-31.
-
26. Weir E, Pretlow T, Pitts A, Williams EE. Destruction of endogenous peroxidase activity in order to locate cellular antigens by peroxidase-labeled antibodies. J Histochem Cytochem. 1974;22:51 – 4.
-
27. Andrew SM, Jasani B. An improved method for the inhibition of endogenous peroxidase non-deleterious to lymphocyte surface markers. Application to immunoperoxidase studies on eosinophil-rich tissue preparations. Histochem J. 1987;19(8):426-30.
-
28. Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, MacDonald S et al. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984;32(2):219-29.
-
29. Wagner L, Worman CP. Color-contrast staining of two different lymphocyte subpopulations: a two-color modification of alkaline phosphatase monoclonal anti-alkaline phosphatase complex technique. Stain Technol. 1988;63(3):129-36.
-
30. Kuhlmann WD, Peschke P. Glucose oxidase as label in histological immunoassays with enzyme-amplification in a two-step technique: coimmobilized horseradish peroxidase as secondary system enzyme for chromogen oxidation. Histochemistry. 1986;85(1):13-7.
-
31. Mason DY, Sammons R. Alkaline phosphatase and peroxidase for double immunoenzymatic labelling of cellular constituents. J Clin Pathol. 1978;31(5):454-60.
-
32. Faulk WP, Taylor GM. An immunocolloid method for the electron microscope. Immunochemistry. 1971;8(11):1081-3.
-
33. Krenács T, Lászik Z, Dobó E. Application of immunogold- silver staining and immunoenzymatic methods in multiple labelling of human pancreatic Langerhans islet cells. Acta Histochem. 1989;85(1):79-85.
-
34. ADL. DP, Mukdsi J, JP. P, Gutiérrez S, AA. Q, CA M et al. Immunoelectron Microscopy: A Reliable Tool for the Analysis of Cellular Processes. En: IntechOpen, editor. Applications of Immunohistochemistry; 2011.
-
35. Park C-H, Kim H, Chang B-J, Lee S, Chang B, Bae C-S et al. Overview of Immunoelectron Microscopy. Appl Microsc. 2018;48:87-95.
-
36. Fraenkel-Conrat H, Olcott HS. The reaction of formaldehyde with proteins; cross-linking between amino and primary amide or guanidyl groups. J Am Chem Soc. 1948;70(8):2673-84.
-
37. Fraenkel-Conrat H, Olcott HS. Reaction of formaldehyde with proteins; cross-linking of amino groups with phenol, imidazole, or indole groups. J Biol Chem. 1948;174(3):827-43.
-
38. Huang SN. Immunohistochemical demonstration of hepatitis B core and surface antigens in paraffin sections. Lab Invest. 1975;33(1):88-95.
-
39. Battifora H, Kopinski M. The influence of protease digestion and duration of fixation on the immunostaining of keratins. A comparison of formalin and ethanol fixation. J Histochem Cytochem. 1986;34(8):1095-100.
-
40. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495-7.
-
41. Taylor CR. The total test approach to standardization of immunohistochemistry. Arch Pathol Lab Med. 2000;124(7):945-51.
-
42. Swaab DF, Pool CW, Van Leeuwen FW. Can specificity ever be proved in immunocytochemical staining. J Histochem Cytochem. 1977;25(5):388-91.
-
43. Fitzgibbons PL, Bradley LA, Fatheree LA, Alsabeh R, Fulton RS, Goldsmith JD et al. Principles of Analytic Validation of Immunohistochemical Assays: Guideline From the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014;138(11):1432-43.
-
44. Fetsch PA, Abati A. Overview of the Clinical Immunohistochemistry Laboratory: Regulations and Troubleshooting Guidelines. En: Javois LC, editor. Immunocytochemical Methods and Protocols. Totowa, NJ: Humana Press; 1999.
-
45. Bass BP, Engel KB, Greytak SR, Moore HM. A review of preanalytical factors affecting molecular, protein, and morphological analysis of formalin-fixed, paraffin-embedded (FFPE) tissue: how well do you know your FFPE specimen? Arch Pathol Lab Med. 2014;138(11):1520- 30.
-
46. Wester K, Wahlund E, Sundström C, Ranefall P, Bengtsson E, Russell PJ et al. Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol. 2000;8(1):61-70.
-
47. Xie R, Chung JY, Ylaya K, Williams RL, Guerrero N, Nakatsuka N et al. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J Histochem Cytochem. 2011;59(4):356-65.
-
48. Leong A, Millos J. An Assessment of the Efficacy of the Microwave Antigen-Retrieval Procedure on a Range of Tissue Antigens. Appl Immunohistochem. 1993;1(4):267-74.
-
49. Shi SR, Imam SA, Young L, Cote RJ, Taylor CR. Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies. J Histochem Cytochem. 1995;43(2):193-201.
-
50. Shi S, Cote R, Taylor C. Standardization and further development of antigen retrieval immunohistochemistry: Strategies and future goals. J Histotechnol. 1999;22:177-92.
-
51. Grabau D, Nielsen O, Hansen SR, Nielsen MM, nkholm A-VL, Knoop A et al., editores. Influence of Storage Temperature and High-Temperature Antigen Retrieval Buffers on Results of Immunohistochemical Staining in Sections Stored for Long Periods; 1998.
-
52. Taylor CR, Shi SR, Chen C, Young L, Yang C, Cote RJ. Comparative study of antigen retrieval heating methods: microwave, microwave and pressure cooker, autoclave, and steamer. Biotech Histochem. 1996;71(5):263-70.
-
53. Heras A, Roach CM, Key ME. Enhanced Polymer Detection System for Immunohistochemistry. Lab Invest. 1995;72(1):A165-A.
-
54. Vyberg M, Nielsen SR, editores. Dextran Polymer Conjugate Two-Step Visualization System for Immunohistochemistry: A Comparison of EnVision+ With Two Three- Step Avidin-Biotin Techniques; 1998.
-
55. Sabattini E, Bisgaard K, Ascani S, Poggi S, Piccioli M, Ceccarelli C et al. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, Chem- Mate, CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51(7):506-11.
-
56. Yaziji H, Taylor CR, Goldstein NS, Dabbs DJ, Hammond EH, Hewlett B et al. Consensus recommendations on estrogen receptor testing in breast cancer by immunohistochemistry. Appl Immunohistochem Mol Morphol. 2008;16(6):513-20.
-
57. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474-81.
-
58. Rhodes A, Jasani B, Balaton AJ, Barnes DM, Miller KD. Frequency of oestrogen and progesterone receptor positivity by immunohistochemical analysis in 7016 breast carcinomas: correlation with patient age, assay sensitivity, threshold value, and mammographic screening. J Clin Pathol. 2000;53(9):688-96.
-
59. Yaziji H, Taylor CR. Begin at the beginning, with the tissue! The key message underlying the ASCO/CAP Taskforce Guideline Recommendations for HER2 testing. Applied immunohistochemistry & molecular morphology : AIMM. 2007;15(3):239-41.
-
60. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118-45.
-
61. Taylor CR, Levenson RM. Quantification of immunohistochemistry– issues concerning methods, utility and semiquantitative assessment II. Histopathology. 2006;49(4):411-24.
-
62. Taylor CR. Quantifiable internal reference standards for immunohistochemistry: the measurement of quantity by weight. Appl Immunohistochem Mol Morphol. 2006;14(3):253-9.
-
63. Herman GE, Elfont EA, Floyd AD. Overview of automated immunostainers. Methods Mol Biol. 1994;34:383- 403.
-
64. Le Neel T, Moreau A, Laboisse C, Truchaud A. Comparative evaluation of automated systems in immunohistochemistry. Clin Chim Acta. 1998;278(2):185-92.
-
65. Moreau A, Néel TL, Joubert M, Truchaud A, Laboisse C. Approach to automation in immunohistochemistry.Clin Chim Acta. 1998;278 2:177-84.
-
66. Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv Anat Pathol. 2017;24(6):311-35.
-
67. Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B et al. Assessing Tumor-infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method From the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma In Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv Anat Pathol. 2017;24(5):235-51.
-
68. Gorris MAJ, Halilovic A, Rabold K, van Duffelen A, Wickramasinghe IN, Verweij D et al. Eight-Color Multiplex Immunohistochemistry for Simultaneous Detection of Multiple Immune Checkpoint Molecules within the Tumor Microenvironment. J Immunol. 2018;200(1):347-54.
-
69. Klauschen F, Müller KR, Binder A, Bockmayr M, Hägele M, Seegerer P, et al. Scoring of tumor-infiltrating lymphocytes: From visual estimation to machine learning. Semin Cancer Biol. 2018;52(Pt 2):151-7.
-
70. H. R. Tumors of the hematopoietic system. In: Atlas of Tumor Pathology. Washington D.C: Armed Institute of Pathology 1966.
-
71. Lukes RJ, Collins RD. Immunologic characterization of human malignant lymphomas. Cancer. 1974;34(4 Suppl):suppl:1488-503.
-
72. National Cancer Institute sponsored study of classifications of non-Hodgkin’s lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin’s Lymphoma Pathologic Classification Project. Cancer. 1982;49(10):2112-35.
-
73. Bernard A, Boumsell L. The clusters of differentiation (CD) defined by the First International Workshop on Human Leucocyte Differentiation Antigens. Hum Immunol. 1984;11(1):1-10.
-
74. Subcommittee I-WN. Nomenclature for clusters of differentiation (CD) of antigens defined on human leukocyte populations. IUIS-WHO Nomenclature Subcommittee. Bull World Health Organ. 1984;62(5):809-15.
-
75. C.R. T, R.J. C. Tumors of unknown origin. En: C.R T, editor. Immunomicroscopy A diagnostic tool for the Surgical Pathologist Major Problems in Pathology. 3 ed: Saunders; 2006.
-
76. D.J. D. Immunohistology of metastatic carcinoma of unknown primary site. En: R. B, D.J. D, editores. Diagnostic immunohistochemistry. Theranostic and Genomic Applications. 5 ed: Elsevier; 2019.
-
77. Hofman V, Lassalle S, Bence C, Long-Mira E, Nahon- Estève S, Heeke S et al. Any Place for Immunohistochemistry within the Predictive Biomarkers of Treatment in Lung Cancer Patients? Cancers (Basel). 2018;10(3):70.
-
78. Gu J, Taylor CR. Practicing pathology in the era of big data and personalized medicine. Applied immunohistochemistry & molecular morphology: AIMM. 2014;22(1):1- 9.
-
79. Taylor CR. Predictive biomarkers and companion diagnostics. The future of immunohistochemistry: “in situ proteomics,” or just a “stain”? Applied immunohistochemistry & molecular morphology : AIMM. 2014;22(8):555- 61.
-
80. Yuan J, Hegde PS, Clynes R, Foukas PG, Harari A, Kleen TO et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4(1):3.
-
81. Fisher B, Redmond C, Brown A, Wickerham DL, Wolmark N, Allegra J et al. Influence of tumor estrogen and progesterone receptor levels on the response to tamoxifen and chemotherapy in primary breast cancer. J Clin Oncol. 1983;1(4):227-41.
-
82. Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 1990;50(14):4177-89.
-
83. Jordan VC. Tamoxifen: a personal retrospective. Lancet Oncol. 2000;1(1):43-9.
-
84. Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561-6.
-
85. Goldman JM, Druker BJ. Chronic myeloid leukemia: current treatment options. Blood. 2001;98(7):2039-42.
-
86. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-82.
-
87. Hanna WM, Hammond E, Taylor CR, Dabbs DJ, Penault- Llorca F, Bloom KJ et al. Re: High concordance between immunohistochemistry and fluorescence in situ hybridization testing for HER2 status in breast cancer requires a normalized IHC scoring system. Mod Pathol. 2008;21(10):1278-80; author reply 80-1.
-
88. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-92.
-
89. Foran JM, Cunningham D, Coiffier B, Solal-Celigny P, Reyes F, Ghielmini M et al. Treatment of mantle-cell lymphoma with Rituximab (chimeric monoclonal anti-CD20 antibody): analysis of factors associated with response. Ann Oncol. 2000;11 Suppl 1:117-21.
-
90. In Vitro Diagnostics [Internet]. U.S. Food and Drug Administration.2016 [consultado 09 noviembre 2020]. Disponible en: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ ucm301431.htm. .
-
91. Matos LLd, Trufelli DC, de Matos MGL, da Silva Pinhal MA. Immunohistochemistry as an important tool in biomarkers detection and clinical practice. Biomark Insights. 2010;5:9-20.
-
92. Nowell PC. The minute chromosome (Phl) in chronic granulocytic leukemia. Blut. 1962;8:65-6.
-
93. J. R. Chromosomes in leukemia and lymphoma. Seminars in Hematology. 1978;15(3):301-19.
-
94. Watson JD, Crick FHC. Genetical Implications of the Structure of Deoxyribonucleic Acid. Nature. 1953;171(4361):964-7.
-
95. Watson JD, Crick FHC. Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid. Nature. 1953;171(4356):737-8.
-
96. van der Ploeg M. Cytochemical nucleic acid research during the twentieth century. Eur J Histochem. 2000;44(1):7-42.
-
97. Smeets DF. Historical prospective of human cytogenetics: from microscope to microarray. Clin Biochem. 2004;37(6):439-46.
-
98. Speicher MR, Carter NP. The new cytogenetics: blurring the boundaries with molecular biology. Nat Rev Genet. 2005;6(10):782-92.
-
99. Penault-Llorca F, Bilous M, Dowsett M, Hanna W, Osamura RY, Rüschoff J, et al. Emerging technologies for assessing HER2 amplification. Am J Clin Pathol. 2009;132(4):539-48.
-
100. Gall JG. The origin of in situ hybridization – A personal history. Methods. 2016;98:4-9.
-
101. Gall JG, Pardue ML. Formation and detection of RNADNA hybrid molecules in cytological preparations. Proc Natl Acad Sci U S A. 1969;63(2):378-83.
-
102. John HA, Birnstiel ML, Jones KW. RNA-DNA hybrids at the cytological level. Nature. 1969;223(5206):582-7.
-
103. Buongiorno-Nardelli M, Amaldi F. Autoradiographic detection of molecular hybrids between RNA and DNA in tissue sections. Nature. 1970;225(5236):946-8.
-
104. Bauman JG, Wiegant J, Borst P, van Duijn P. A new method for fluorescence microscopical localization of specific DNA sequences by in situ hybridization of fluorochromelabelled RNA. Exp Cell Res. 1980;128(2):485- 90.
-
105. Wilcox JN. Fundamental principles of in situ hybridization. J Histochem Cytochem. 1993;41(12):1725-33.
-
106. Jensen E. Technical review: In situ hybridization. Anat Rec (Hoboken). 2014;297(8):1349-53.
-
107. Tanner M, Gancberg D, Di Leo A, Larsimont D, Rouas G, Piccart MJ et al. Chromogenic in situ hybridization: a practical alternative for fluorescence in situ hybridization to detect HER-2/neu oncogene amplification in archival breast cancer samples. Am J Pathol. 2000;157(5):1467- 72.
-
108. Park K, Han S, Kim J-Y, Kim H-J, Kwon JE, Gwak G. Silver-Enhanced In Situ Hybridization as an Alternative to Fluorescence In Situ Hybridization for Assaying HER2 Amplification in Clinical Breast Cancer. J Breast Cancer. 2011;14(4):276-82.
-
109. Pedersen M, Rasmussen BB. The correlation between dual-color chromogenic in situ hybridization and fluorescence in situ hybridization in assessing HER2 gene amplification in breast cancer. Diagn Mol Pathol. 2009;18(2):96-102.
-
110. Gulley ML, Glaser SL, Craig FE, Borowitz M, Mann RB, Shema SJ et al. Guidelines for interpreting EBER in situ hybridization and LMP1 immunohistochemical tests for detecting Epstein-Barr virus in Hodgkin lymphoma. Am J Clin Pathol. 2002;117(2):259-67.
-
111. Cook JR. Paraffin section interphase fluorescence in situ hybridization in the diagnosis and classification of nonhodgkin lymphomas. Diagn Mol Pathol. 2004;13(4):197- 206.
-
112. Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94(5):604-16.
-
113. Ting C-Y, Chang K-M, Kuan J-W, Sathar J, Chew L-P, Wong O-LJ et al. Clinical Significance of BCL2, C-MYC, and BCL6 Genetic Abnormalities, Epstein-Barr Virus Infection, CD5 Protein Expression, Germinal Center B Cell/Non-Germinal Center B-Cell Subtypes, Co-expression of MYC/BCL2 Proteins and Co-expression of MYC/ BCL2/BCL6 Proteins in Diffuse Large B-Cell Lymphoma: A Clinical and Pathological Correlation Study of 120 Patients. Int J Med Sci. 2019;16(4):556-66
-
114. Ma Z, Niu J, Cao Y, Pang X, Cui W, Zhang W et al. Clinical significance of ‘double-hit’ and ‘double-expression’ lymphomas. J Clin Pathol. 2020;73(3):126-38.
-
115. Thunnissen E, Bubendorf L, Dietel M, Elmberger G, Kerr K, Lopez-Rios F, et al. EML4-ALK testing in non-small cell carcinomas of the lung: a review with recommendations. Virchows Arch. 2012;461(3):245-57.
-
116. Schildhaus HU, Deml KF, Schmitz K, Meiboom M, Binot E, Hauke S, et al. Chromogenic in situ hybridization is a reliable assay for detection of ALK rearrangements in adenocarcinomas of the lung. Mod Pathol. 2013;26(11):1468-77.
-
117. Luk PP, Selinger CI, Cooper WA, Mahar A, Palme CE, O’Toole SA, et al. Clinical Utility of In Situ Hybridization Assays in Head and Neck Neoplasms. Head Neck Pathol. 2019;13(3):397-414.
-
118. Ferrara G, De Vanna AC. Fluorescence In Situ Hybridization for Melanoma Diagnosis: A Review and a Reappraisal. Am J Dermatopathol. 2016;38(4):253-69.
-
119. Tanas MR, Goldblum JR. Fluorescence in situ hybridization in the diagnosis of soft tissue neoplasms: a review. Adv Anat Pathol. 2009;16(6):383-91.
-
120. Nitta H, Hauss-Wegrzyniak B, Lehrkamp M, Murillo AE, Gaire F, Farrell M et al. Development of automated brightfield double in situ hybridization (BDISH) application for HER2 gene and chromosome 17 centromere (CEN 17) for breast carcinomas and an assay performance comparison to manual dual color HER2 fluorescence in situ hybridization (FISH). Diagn Pathol. 2008;3:41121.
-
121. Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975;94(3):441-8.
-
122. Heather JM, Chain B. The sequence of sequencers: The history of sequencing DNA. Genomics. 2016;107(1):1-8.
-
123. Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263-73.
-
124. Mullis KB, Faloona FA. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335-50.
-
125. Mullis KB. The unusual origin of the polymerase chain reaction. Sci Am. 1990;262(4):56-61, 4-5.
-
126. Nathwani BN, Sasu SJ, Ahsanuddin AN, Hernandez AM, Drachenberg MR. The critical role of histology in an era of genomics and proteomics: a commentary and reflection. Adv Anat Pathol. 2007;14(6):375-400.
-
127. Eltoum IA, Siegal GP, Frost AR. Microdissection of histologic sections: past, present, and future. Adv Anat Pathol. 2002;9(5):316-22.
-
128. Hunt JL, Finkelstein SD. Microdissection techniques for molecular testing in surgical pathology. Arch Pathol Lab Med. 2004;128(12):1372-8.
-
129. Espina V, Heiby M, Pierobon M, Liotta LA. Laser capture microdissection technology. Expert Rev Mol Diagn. 2007;7(5):647-57.
-
130. Going J. Histological microdissection in diagnostic and investigative pathology. Diagnostic Histopathology. 2010;16:43-8.
-
131. Goelz SE, Hamilton SR, Vogelstein B. Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem Biophys Res Commun. 1985;130(1):118-26.
-
132. Bianchi AB, Navone NM, Conti CJ. Detection of loss of heterozygosity in formalin-fixed paraffin-embedded tumor specimens by the polymerase chain reaction. Am J Pathol. 1991;138(2):279-84. 133. Going JJ, Lamb RF. Practical histological microdissection for PCR analysis. J Pathol. 1996;179(1):121-4.
-
134. Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR et al. Laser capture microdissection. Science. 1996;274(5289):998-1001.
-
135. Gross A, Schoendube J, Zimmermann S, Steeb M, Zengerle R, Koltay P. Technologies for Single-Cell Isolation. Int J Mol Sci. 2015;16(8):16897-919.
-
136. d’Amore F, Stribley JA, Ohno T, Wu G, Wickert RS, Delabie J et al. Molecular studies on single cells harvested by micromanipulation from archival tissue sections previously stained by immunohistochemistry or nonisotopic in situ hybridization. Lab Invest. 1997;76(2):219-24.
-
137. Fend F, Raffeld M. Laser capture microdissection in pathology. J Clin Pathol. 2000;53(9):666-72.
-
138. Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010;16(9):398-406.
-
139. Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781-91.
-
140. Francis G, Stein S. Circulating Cell-Free Tumour DNA in the Management of Cancer. Int J Mol Sci. 2015;16(6):14122-42.
-
141. Finotti A, Allegretti M, Gasparello J, Giacomini P, Spandidos DA, Spoto G et al. Liquid biopsy and PCR-free ultrasensitive detection systems in oncology (Review). Int J Oncol. 2018;53(4):1395-434.
-
142. Fernández-Lázaro D, Hernández JLG, García AC, Castillo ACD, Hueso MV, Cruz-Hernández JJ. Clinical Perspective and Translational Oncology of Liquid Biopsy. Diagnostics (Basel).2020;10(7):443.
-
143. Cappelletti V, Appierto V, Tiberio P, Fina E, Callari M, Daidone MG. Circulating Biomarkers for Prediction of Treatment Response. J Natl Cancer Inst Monogr. 2015;2015(51):60-3.
-
144. von Bubnoff N. Liquid Biopsy: Approaches to Dynamic Genotyping in Cancer. Oncol Res Treat. 2017;40(7- 8):409-16.
-
145. Arneth B. Update on the types and usage of liquid biopsies in the clinical setting: a systematic review. BMC Cancer. 2018;18(1):527.
-
146. Bennett CW, Berchem G, Kim YJ, El-Khoury V. Cell-free DNA and next-generation sequencing in the service of personalized medicine for lung cancer. Oncotarget. 2016;7(43):71013-35.
-
147. Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570(7761):385-9.
-
148. Wang J, Chang S, Li G, Sun Y. Application of liquid biopsy in precision medicine: opportunities and challenges. Front Med. 2017;11(4):522-7.
-
149. Alimirzaie S, Bagherzadeh M, Akbari MR. Liquid biopsy in breast cancer: A comprehensive review. Clin Genet. 2019;95(6):643-60.
-
150. Koch C, Kuske A, Joosse SA, Yigit G, Sflomos G, Thaler S et al. Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity. EMBO Mol Med. 2020;12(9):e11908.
-
151. Johann DJ, Jr., Steliga M, Shin IJ, Yoon D, Arnaoutakis K, Hutchins L et al. Liquid biopsy and its role in an advanced clinical trial for lung cancer. Exp Biol Med (Maywood). 2018;243(3):262-71.
-
152. Couraud S, Vaca-Paniagua F, Villar S, Oliver J, Schuster T, Blanché H et al. Noninvasive diagnosis of actionable mutations by deep sequencing of circulating free DNA in lung cancer from never-smokers: a proof-of-concept study from BioCAST/IFCT-1002. Clin Cancer Res. 2014;20(17):4613-24.
-
153. Wills B, Gorse E, Lee V. Role of liquid biopsies in colorectal cancer. Curr Probl Cancer. 2018;42(6):593-600.
-
154. Marcuello M, Vymetalkova V, Neves RPL, Duran-Sanchon S, Vedeld HM, Tham E et al. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol Aspects Med. 2019;69:107-22.
-
155. Gao Y, Zhu Y, Yuan Z. Circulating Tumor Cells and Circulating Tumor DNA Provide New Insights into Pancreatic Cancer. Int J Med Sci. 2016;13(12):902-13.
-
156. Gray ES, Reid AL, Bowyer S, Calapre L, Siew K, Pearce R, et al. Circulating Melanoma Cell Subpopulations: Their Heterogeneity and Differential Responses to Treatment. J Invest Dermatol. 2015;135(8):2040-8.
-
157. Strotman LN, Millner LM, Valdes R, Jr., Linder MW. Liquid Biopsies in Oncology and the Current Regulatory Landscape. Mol Diagn Ther. 2016;20(5):429-36.
-
158. Siravegna G, Mussolin B, Venesio T, Marsoni S, Seoane J, Dive C et al. How liquid biopsies can change clinical practice in oncology. Ann Oncol. 2019;30(10):1580-90.
-
159. Rossi G, Ignatiadis M. Promises and Pitfalls of Using Liquid Biopsy for Precision Medicine. Cancer Res. 2019;79(11):2798-804.
Recibido: Noviembre 13, 2020
Aceptado: Diciembre 15, 2020
Correspondencia:
María del Pilar
Archila-Gomez archilapilar@yahoo.com