Abstract
Introduction. Coronavirus disease 2019 (COVID-19) is characterized by generating adult respiratory distress syndrome and multi-organ dysfunction, but it also has a prothrombotic effect that must be considered for the correct approach with patients.
Clinical case. 50-year-old patient who consulted due to a 4-day clinical febrile syndrome and pain in the lower abdomen. In the review by systems, he referred dysgeusia, anosmia and occasional dry cough.
Initial paraclinical tests showed, polyglobulia, thrombocytosis, elevated D-dimer, and arterial gases with respiratory alkalemia. Chest computed tomography (CT) was compatible with viral pneumonia and the contrast abdominal CT had evidence of mesenteric ischaemia. Intraoperative findings of multiple thrombi at the mesentery level.
Discussion. The prothrombotic state induced by COVID-19 has been predominantly described in the venous system, but we must not ignore its arterial involvement even in unusual sites, since if it is not suspected it can generate fatal consequences for patients.
Keywords: Mesenteric Ischemia; COVID-19; SARS-CoV-2
Isquemia Mesentérica y Covid-19. Reporte de caso
Resumen
Introducción. La enfermedad por coronavirus 2019 (COVID-19) se caracteriza por generar síndrome de dificultad respiratoria del adulto y disfunción multiorgánica, sin embargo, tiene un efecto protrombótico que se debe tener en cuenta para un adecuado abordaje de los pacientes.
Presentación del caso: Individuo masculino de 50 años, asiste por clínica de 4 días de fiebre y dolor en hemiabdomen inferior.
En la revisión por sistemas reveló disgeusia, anosmia y tos seca ocasional. Exámenes paraclínicos iniciales reportaron poliglobulia, trombocitosis, dímero D elevado y gases arteriales con alcalemia respiratoria.
La tomografía de tórax compatible con neumonía viral y tomografía de abdomen contrastada con evidencia de isquemia mesentérica. Los hallazgos intraoperatorios fueron múltiples trombos a nivel del mesenterio.
Discusión. El estado protrombótico inducido por la COVID-19 se ha descrito predominantemente en el sistema venoso, pero no se debe dejar de lado su compromiso arterial, ya que si no se sospecha puede generar consecuencias fatales para los pacientes.
Palabras clave: Isquemia Mesentérica; COVID-19; SARS-CoV-2.
Introduction – Mesenteric Ischemia and Covid-19
The first pandemic of the century; COVID-19 (Coronavirus 2019) caused by the new SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), a virus belonging to the Coronaviridae family of the B-coronavirus subgroup (1), is currently recognized worldwide for the high mortality secondary to the systemic inflammatory reaction and the adult respiratory distress syndrome that it generates.
Following the Middle East respiratory syndrome (MERS-CoV) and the severe acute respiratory syndrome (SARS-CoV), SARS-CoV-2 is the third coronavirus to cause infection worldwide in the last 20 years.
In a study conducted in Milan, Italy, a general decrease in emergency surgical procedures was identified in 2020 compared to 2019, however, an increase was identified in certain procedures, within which a significant increase was found (p= 0,002) in the interventions secondary to acute mesenteric ischaemia.
Although the authors do not describe a clear explanation (2), they propose as possible hypotheses, the participation of the thrombogenic effect of COVID-19 or the possible dietary changes during the pandemic. To date, there is some data on mesenteric ischaemia in COVID-19, extracted from several published cases and grouped through systematic reviews (3,4).
The following is a case of an unusual thromboembolic event manifested by mesenteric thrombosis in a patient with positive reverse transcription-polymerase chain reaction (RT-PCR) for SARS-CoV-2.
Clinical Case – Mesenteric Ischemia and Covid-19
A 50-year-old overweight male from a rural area with otherwise unremarkable past medical history, consulted due to a four day history of fever with peaks quantified up to 39° Celsius (C), unresponsive to antipyretics.
At 48 hours after the onset of fever, he presented continuous abdominal pain, located in the lower quadrants of moderate intensity, aggravated with food intake, and without improvement after self-prescribed intramuscular administration of non-steroidal anti-inflammatory drugs. Additionally, presented numerous foamy and non-bloody diarrheal stools, that spontaneously resolved.
Later the pain intensifies and generalizes to the entire abdomen, for which he consults to the emergency department. When inquiring into the systems review, the presence of anosmia, dysgeusia, and occasional non-cyanotic or emetizing dry cough were established, which began two days before the consultation.
Physical examination on admittance, revealed the patient was tachycardic (120 beats per minute), normotensive, with a fever of 37.5 °C axillary, tachypneic (25 breaths per minute), with adequate oxygen saturation at room air, and a regular general condition with grimacing.
Cardiopulmonary without alterations, flat abdomen, gastrointestinal sounds present, normotympanic, painful on deep and superficial palpation, with generalized muscular defense, and a rebound tenderness in all four quadrants.
Laboratory values indicated polyglobulia and thrombocytosis in the hemogram, elevated D-dimer, arterial gases with respiratory alkalemia, without oxygenation disorder, and hyperlactatemia, with amylase levels three times higher than the normal value, and positive RT-PCR for SARS-CoV-2.
Imaging studies with high resolution chest computed tomography (CT) identified predominantly peripheral ground glass pattern in both lung fields compatible with viral pneumonia (Figure 1) and; the contrast abdominal CT showed diffuse hepatic steatosis, concentric and diffuse thickening of the proximal ileum walls reaching a maximum thickness of 12 millimeters (mm) in a long segment of approximately 30 centimeters (cm), engorgement of mesenteric vessels, inflammatory changes in fat, opacification defects corresponding to thrombi in the mesenteric artery, 1 cm above from its origin occluding approximately 60% of the vessel’s lumen in a segment with a length of 12 mm and another more distal at the height of the ileal arteries branching that occludes approximately 80% of the vessel’s lumen, and dilation of jejunal and ileal thin intestinal loops reaching a maximum transverse diameter of 38 mm, no transitional areas were observed (Figure 2).
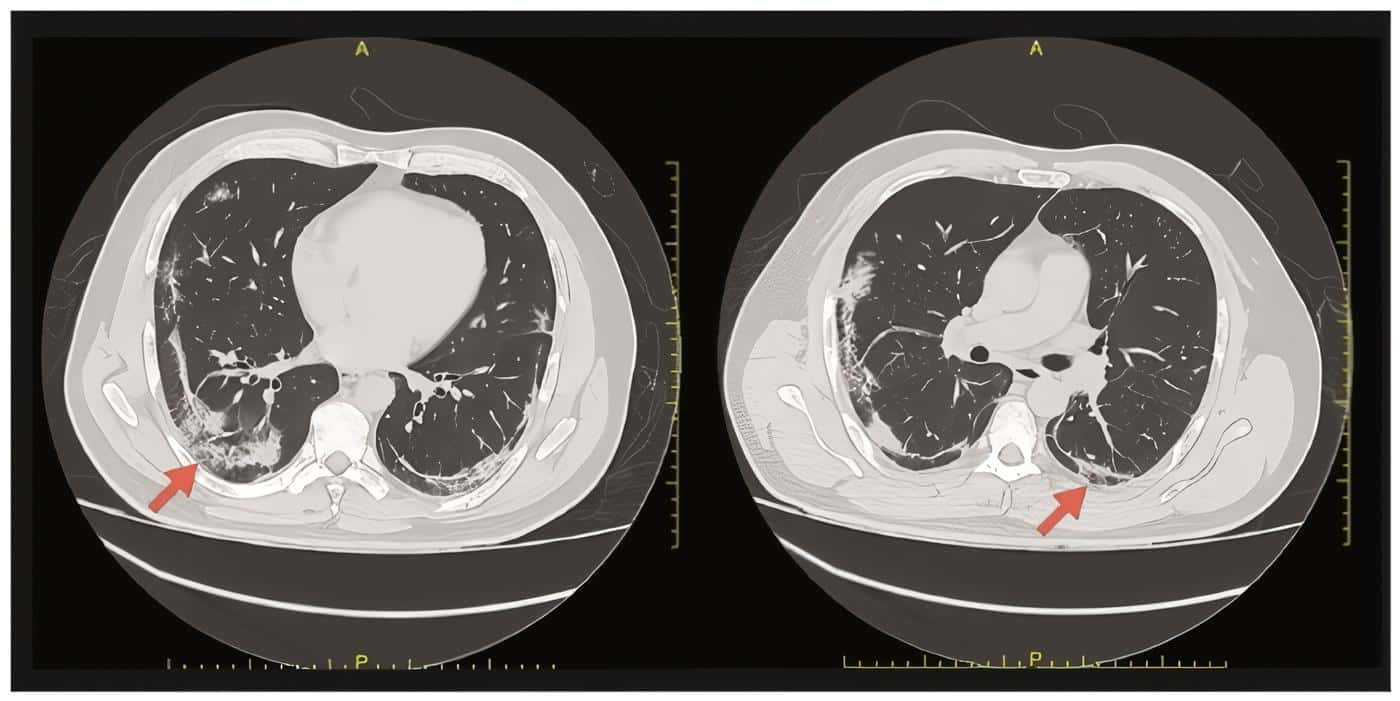
Figure 1. High-resolution chest computed tomography. Axial view. The arrows show areas in both lung fields of subpleural ground glass pattern and basal subsegmentary atelectasis. Findings are suggestive of viral pneumonia secondary to COVID-19.
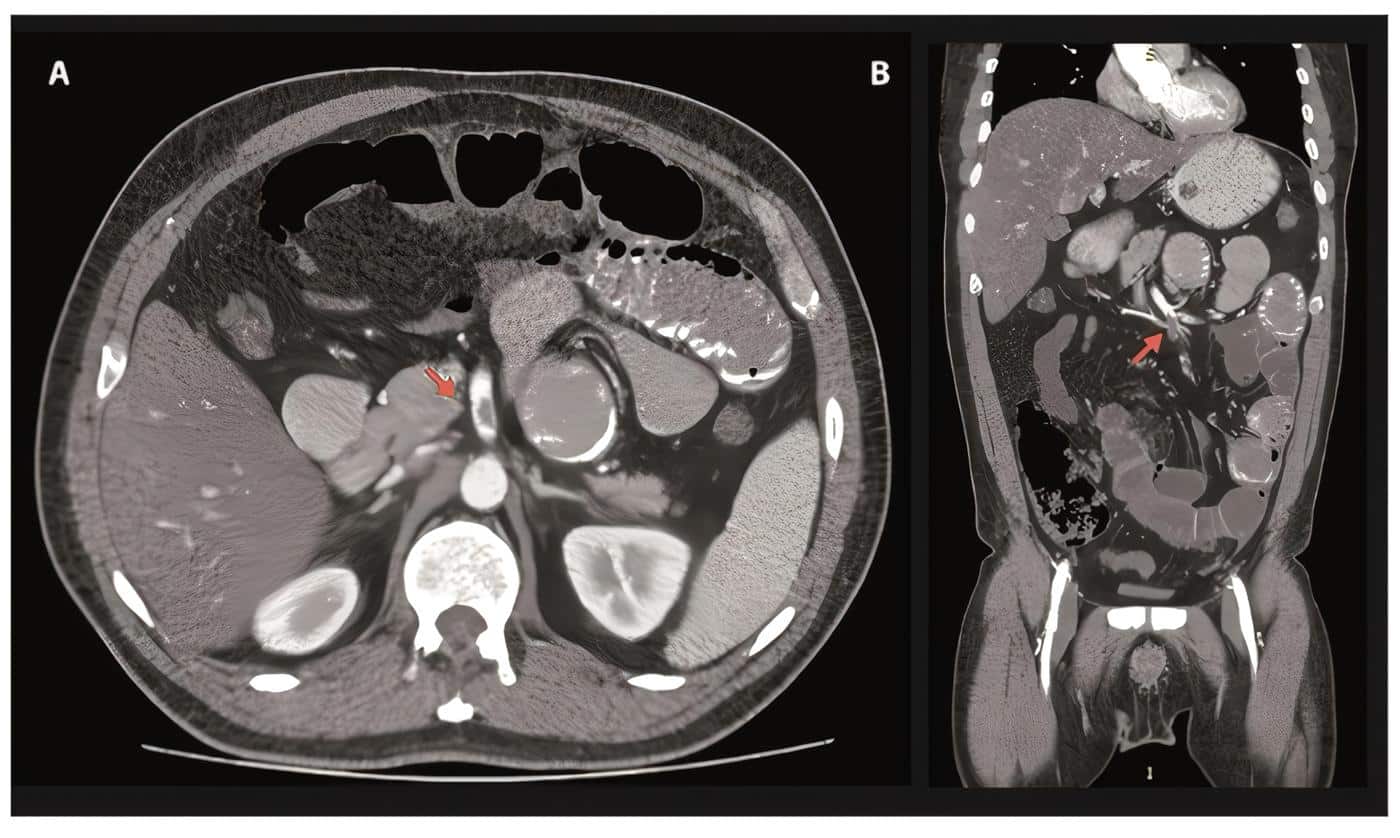
Figure 2. Contrast abdominopelvic computed angiotomography A. Axial view. The arrow indicates the location of the opacification defect, which corresponds to thrombi in the mesenteric artery, 1cm above its origin, occluding approximately 60% of the vessel’s lumen. B. Coronal Cut. The arrow indicates branching of the ileal arteries where the thrombi occludes approximately 80% of the vessel’s lumen.
The patient was taken to emergency laparotomy, with intraoperative findings of 200 cubic centimeters of peritoneal fluid with evidence of perforation, additionally a 40 cm area of ischaemia at the level of the ileum at 80 cm from the ligament of the Treitz angle, with multiple thrombi to level of the mesentery, for which they performed a 40-cm intestinal resection with an end-to-end ileal ileus anastomosis plus peritoneal lavage and abdominal wall closure.
He required surveillance in the intensive care unit (ICU) and later in a general ward, with analgesia and from the respiratory point of view only required oxygen through a nasal cannula at 2 liters per minute. He was discharged after 7 days, with indication of outpatient anticoagulation with low molecular weight heparins.
Discussion – Mesenteric Ischemia and Covid-19
This study describes the clinical evolution of a case of mesenteric ischaemia in a patient who underwent RT-PCR for SARS-CoV-2 with a positive result during hospitalization. Upon admission, COVID-19 infection was not suspected, however, upon review of systems, symptoms suggestive of mild respiratory infection were found.
This proposes that in the context of a pandemic, it is necessary to systematically carry out a complete interrogation and physical examination to guide a possible positive case and to avoid possible complications associated with thrombosis.
During the course of the current pandemic, a great effort has been devoted to recognize and manage SARS-CoV-2 infection, dedicating greater impetus to respiratory symptoms. However, various systemic complications have been demonstrated, highlighting that there is currently wide evidence suggesting a state of hypercoagulability (5).
COVID-19 has been considered a phenomenon that induces autoimmune response associated with higher titers of antiphospholipid antibodies, suggesting a theory of latent autoimmunity accountable for clinical outcomes(6).
Ignat et al. (7) reported a series of 3 cases of hospitalized patients (2 men and 1 woman), positive for SARS-CoV-2 who developed intestinal ischaemia:
In which two of them had normal clinical examination, but because of deterioration in the clinical course, they decided to perform a contrast abdomen CT, which corroborated the diagnosis. Cheung et al. (8) reported one of the first cases of mesenteric ischaemia in a patient with COVID 19, which, like the present case, was a man who exhibited absence of respiratory symptoms and acute abdominal pain.
Vulliamy et al. (9) display another case in a man, who, unlike the present case, debuted with mild respiratory compromise in the two weeks prior to abdominal symptoms, subsequently documenting aortic thrombosis with embolism to the mesenteric artery, also requiring extraction surgery of the ischaemic segment of the intestine.
Kaafarani et al. (10) in a series that included 141 SARS-CoV-2 positive patients who described gastrointestinal complications of which 3.5% were intestinal ischaemia.
It is interesting how in a systematic review carried out by Pirola et al.(3) revealed that none of the evaluated studies reported a history of systemic atherosclerosis or any condition that explained mesenteric ischaemia, similar to that reported by Patel et al., who suggested the need to increase research regarding the classification of patients with COVID-19 and suspected intestinal ischaemia(4).
Several hypotheses have been proposed to explain why SARS-CoV-2 infection can cause vascular and / or gastrointestinal injury, which appear to be related to the inflammatory response and intrinsic properties of the virus (11,12).
Angiotensin converting enzyme 2 is the functional receptor of the virus, present in the lung, cardiovascular system, intestine, placenta, among others is overexpressed by the virus, which allows greater penetration of this in digestive cells.
The pathophysiological mechanisms involved are a pro-coagulant, proinflammatory and anti-fibrinolytic state, a consequence of damage at the cellular level; as well as the secondary anti-phospholipid syndrome (13).
This is explained by the infection of the pulmonary vascular bed, the subsequent massive endothelial apoptosis and the activation of the immune response, where monocytes, macrophages and neutrophils participate, with the release of cytokines (Interleukin [IL] 6, IL 1 and Tumor Necrosis Factor).
In a series of autopsies performed during SARS-CoV in 2003, showed signs of diffuse alveolar damage with atypical pneumocytes, as well as signs of diffuse micro-thrombosis at the peripheral level. On the other hand, autopsies carried out in Germany on positive cases for COVID-19 evidenced a prevalence of 21% of pulmonary arterial embolism; establishing this entity as the second cause of death in this population after pneumonia (14).
Other triggers linked to thromboembolic events have been identified, including the hypoxic state, which activates transcription factors such as Activator Protein-1 complex, Early growth response-1 protein, Nuclear Factor kappa-light-chain-enhancer of activated B cells, and Hypoxia-Induced factors (15).
The understanding of this pro-coagulant cascade added to the knowledge of the markers evaluated in clinical practice (fibrinogen, D-dimer, platelet count, clotting times), allow us to suspect a possible outcome in the management of these patients. Several studies have described that the presence of these high markers or in the upper limit usually have a fatal outcome (Table 1).
Table 1. Risk factors associated with COVID-19 and thrombosis
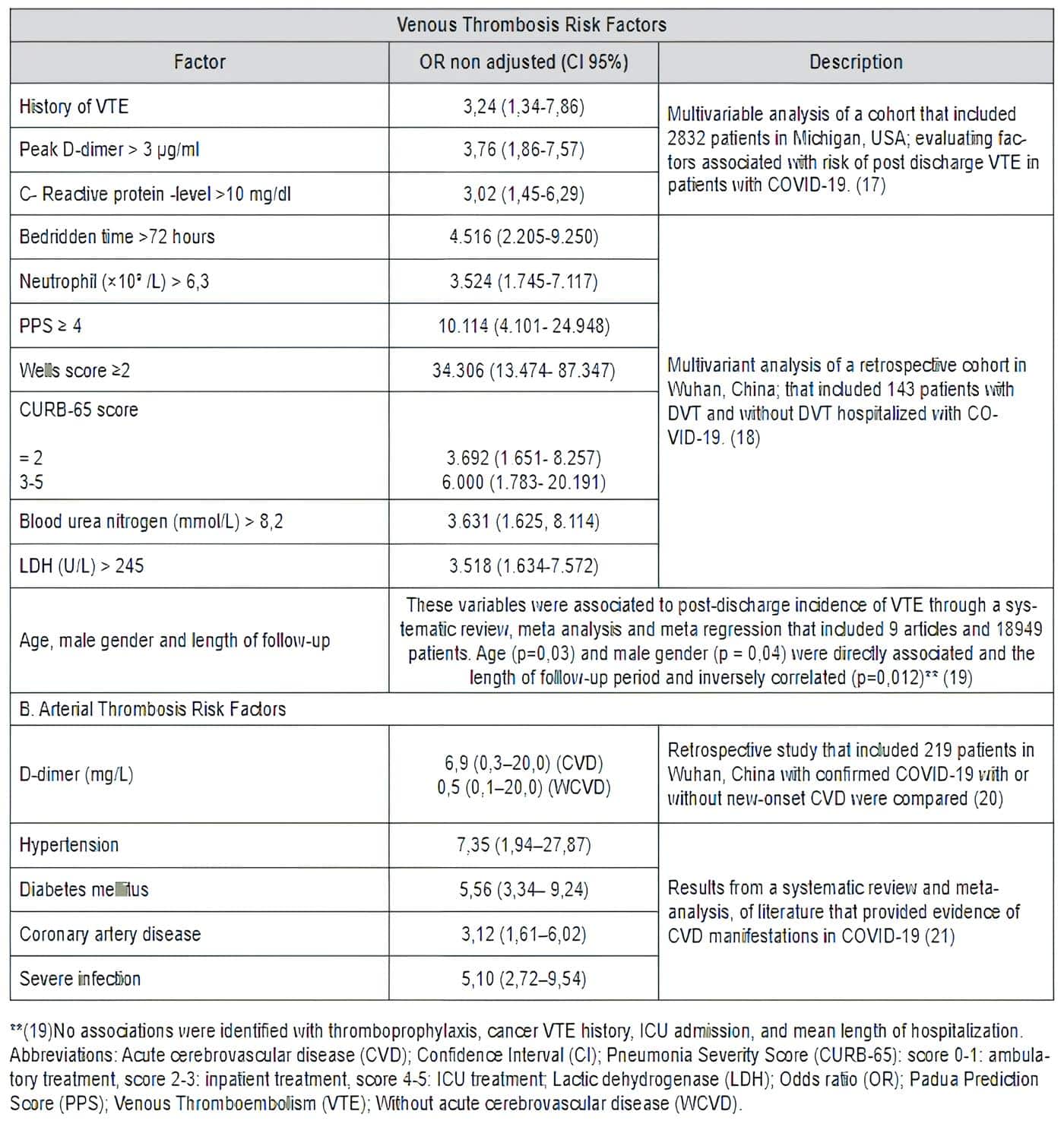
It has been described that patients with COVID-19 who have high D-dimer values are more likely to require admission to the ICU, non-invasive mechanical ventilation or death and it is attributed to a hyperimmune response of the host (12,16).
Acute mesenteric ischaemia secondary to thrombosis in the presence of SARS-CoV-2 infection can be explained by the hypercoagulable state attributed to the infection and should be suspected in patients with confirmed or suspected infection and gastrointestinal symptoms (5), to give prompt treatment and avoid fatal consequences.
References – Mesenteric Ischemia and Covid-19
1.Joint WHO-China Study. WHO-convened Global Study of Origins of SARS-CoV-2: China Part. [Internet]. Who. int. 2022 [consultado 26 octubre 2021]. Disponible en:
https://www.who.int/publications/i/item/who-convened-global-study-of-origins-of-sars-cov-2-china-part
2. Kurihara H, Marrano E, Ceolin M, Chiara O, Faccincani R, Bisagni P, et al. Impact of lockdown on emergency general surgery during first 2020 COVID-19 outbreak. European journal of trauma and emergency surgery . 2021;47(3):677–82.
3. Pirola L, Palermo A, Mulinacci G, Ratti L, Fichera M, Invernizzi P, et al. Acute mesenteric ischemia and small bowel imaging findings in COVID-19: A comprehensive review of the literature. World Journal of Gastrointestinal Surgery. 2021;13(7):702–16.
4. Patel S, Parikh C, Verma D, Sundararajan R, Agrawal U, Bheemisetty N, et al. Bowel ischemia in COVID-19: A systematic review. International Journal of Clinical Practice. 2021;75(12).
5. Kerawala AA, Das B, Solangi A. Mesenteric ischemia in COVID-19 patients: A review of current literature. World Journal of Clinical Cases. 2021;9(18):4700–8.
6. Anaya JM, Monsalve DM, Rojas M, Rodríguez Y, Montoya-García N, Mancera-Navarro LM, et al. Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19. Journal of Translational Autoimmunity. 2021;4:100091
7. Ignat M, Philouze G, Aussenac-Belle L, Faucher V, Collange O, Mutter D, et al. Small bowel ischemia and SARS-CoV-2 infection: an underdiagnosed distinct clinical entity. Surgery 2020;168(1):14–6.
Bibliographies – Mesenteric Ischemia and Covid-19
8. Cheung S, Quiwa JC, Pillai A, Onwu C, Tharayil ZJ, Gupta R. Superior mesenteric artery thrombosis and acute intestinal ischemia as a consequence of COVID-19 infection. American Journal of Case Reports. 2020;21:1–3.
9. Vulliamy P, Jacob S, Davenport RA. Acute aorto-iliac and mesenteric arterial thromboses as presenting features of COVID-19. British Journal of Haematology. 2020;489(6):1053–4.
10. Kaafarani HMA, el Moheb M, Hwabejire JO, Naar L, Christensen MA, Breen K, et al. Gastrointestinal Complications in Critically Ill Patients With COVID-19. Annals of surgery. 2020;272(2):e61–2.
11. Estevez-Cerda SC, Saldaña-Rodríguez JA, Alam-Gidi AG, Riojas-Garza A, Rodarte-Shade M, Velazco-de la Garza J, et al. Severe bowel complications in SARSCoV-2 patients receiving protocolized care. Revista de Gastroenterologia de Mexico. 2021;86(4):378-386.
12. López-Reyes R, Oscullo G, Jiménez D, Cano I, García-Ortega A. Thrombotic Risk and Covid-19: Review of Current Evidence for a Better Diagnostic and Therapeutic Approach. Archivos de Bronconeumologia.2021;57:55–64.
13. Guillet H, Gallet R, Pham V, D’humières T, Huguet R, Lim P, et al. Clinical spectrum of ischaemic arterial diseases associated with COVID-19: a series of four illustrative cases. European Heart Journal – Case Reports.;5(1):ytaa488.
14. Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, et al. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. International Journal of Legal Medicine. 2020;134(4):1275–84.
Recommended Readings – Mesenteric Ischemia and Covid-19
15. Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia [Internet]. Journal Of Applied Physiology. 2000 [consultado 26 octubre 2021]. Disponible en: http://www.jap.org
16. Ortega-Paz L, Capodanno D, Montalescot G, Angiolillo DJ. Coronavirus disease 2019–associated thrombosis and coagulopathy: Review of the pathophysiological characteristics and implications for antithrombotic management. Journal of the American Heart Association. 2021;10(3):1–24.
17. Li P, Zhao W, Kaatz S, Latack K, Schultz L, Poisson L. Factors Associated With Risk of Postdischarge Thrombosis in Patients With COVID-19. JAMA Network Open. 202122;4(11):e2135397.
18. Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, et al. Deep Vein Thrombosis in Hospitalized Patients with COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation. 2020;142(2):114–28.
19. Zuin M, Engelen MM, Barco S, Spyropoulos AC, Vanassche T, Hunt BJ, et al. Incidence of venous thromboembolic events in COVID-19 patients after hospital discharge: A systematic review and meta-analysis. Thrombosis Research. 2022;209:94–8.
20. Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke and Vascular Neurology.2020;5(3):279–84.
21. Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: A systematic review and meta-analysis. International Journal of Stroke. 2021;16(2):137–49.
Authors – Mesenteric Ischemia and Covid-19
1 Ana María Pérez Murcia. Servicio de Medicina Interna, Hospital de San José. Especialista Docencia Universitaria. Fundación Universitaria de Ciencias de la Salud.
2 Paula Daniela Nieto-Zambrano, Héctor Fabio Restrepo, Adriana Rojas-Villarraga. Vicerrectoría de investigaciones. Fundación Universitaria de ciencias de la Salud.
3 Carlos Alfonso Madariaga Carocci, Servicio de Medicina Interna, Hospital de San José. Fundación Universitaria de Ciencias de la Salud.
Recibido: 27 de enero de 2022
Aceptado: 7 de marzo de 2022
Correspondencia:
Paula Daniela Nieto-Zambrano
pdnieto@fucsalud.edu.co